L-lysine and vitamin C work better in synergy against Escherichia coli and Acinetobacter baumanniis
For centuries various plants and their natural compounds were used as antimicrobial agents. Most research work in this field has been done with individual compounds and directed towards their mechanisms of action or clinical applications. The interest in natural anti-microbial approaches has intensified in recent years as extensive use of antibiotics, drugs and food antimicrobials have led to the occurrence of resistant microbial strains. Our research aims at increasing efficacy of natural compounds through their specific combinations, that allow for simultaneously affecting cellular mechanisms of interest and increasing efficacy at lower doses than individual compounds. Here we present results of the study that revealed that combination of vitamin C and L-lysine, which individually do not show such efficacy, have bacteriostatic and bactericidal effect against two Gram-negative strains Acinetobacter baumannii and Escherichia coli.
The intensive use of antibiotics, drugs, and food antimicrobials has led to the occurrence of resistant microbial strains that limit effectiveness of current medical or biotechnological approaches.¹ Among many bacterial strains Acinetobacter baumannii (A. baumannii), primarily a nosocomial pathogen, is one of the most antibiotic resistant pathogens in clinical medicine.2,3 It can cause infections in the blood, urinary tract, or lungs (pneumonia). It can also “colonize” or live in a patient without causing infections or symptoms, especially in respiratory secretions (sputum) or open wounds. Estimates of mortality rates among patients with A. baumannii infections have ranged from 26.0% to 55.7%, with estimated attributable mortality rates between 8.4% and 36.5%.4 Escherichia coli (E. coli) is a part of normal intestinal flora but can also cause intestinal illness, urinary tract infection, abdominal and pelvic infection, pneumonia, bacteremia, and meningitis, among others. Antibiotic resistance to E. coli is also a concern as it is the most common Gram-negative pathogen in humans. Each year in the United States, E. coli infections cause approximately 265,000 illnesses and about 100 deaths.
Our previous work included designing specific combinations of natural compounds to simultaneously target various cellular mechanisms as means to increase their biological efficacy. Here we used L-lysine and vitamin C to evaluate their combined anti-bacterial efficacy considering that these nutrients are also affecting other mechanisms relevant in curbing infections. Both nutrients are essential for synthesis and structure of collagen and extracellular matrix (ECM) components with an established important role in protecting integrity of biological barriers, which in turn are the entry points for various infection agents. In this aspect, integrity of the endothelial lining of blood vessels, intestines, lung passages and many other organs largely depends on optimum intake of L-lysine and vitamin C as these nutrients are not produced in a human body.
L-lysine has been used for an antibacterial activity in a form of a polymer, i.e., poly-β-l-lysine, which due to its positively charged cationic groups has shown to be effective in killing bacteria by destroying their membranes. However, its use is limited due to its high cytotoxicity to mammalian cells.5 Antimicrobial efficacy of L-lysine evaluated in disk diffusion test by Svediene et al. showed no inhibition of E. coli growth at 1.0 mg/ml, while its minimal inhibitory concentration (MIC value) against E. coli and S. aureus were 500 mg/ml and 125 mg/ml, respectively. L-lysine did not show efficacy against C. albicans and T. rubrum as well.6
Vitamin C is another widely used antimicrobial compound known since the 1930s with its potent antioxidant, immunomodulatory, and anti-infectious effects.7 The antibacterial effects of vitamin C are, at least in part, due to its low pH and thus milieu-modifying properties. Notably, vitamin C can inhibit the growth of S. aureus and streptococci even under neutral pH conditions.8 It has been shown that vitamin C at 0.31 mg/ml concentrations could inhibit Pseudomonas aeruginosa growth in vitro and its application at low concentration (0.15 mg/ml) could inhibit the growth of Staphylococcus aureus,9 indicating that antibacterial effects of vitamin C might be both bacterial strain and concentration dependent. Interestingly, vitamin C had only a marginal effect on the growth of E. coli ATTC 11775 strain.10 However, vitamin C in combination with lactic acid inhibited replication of E. coli O157:H7 strain when incubated in Brain Heart Infusion (BHI) broth or in carrot juice,11 whereas another study reported that vitamin C reduced the sensitivity of E. coli MG1655 to streptomycin.12 Also, co-administration of vitamin C could sufficiently enhance the antibacterial effects of other agents such as epigallocatechin gallate directed even against multidrug-resistant MRSA bacterial species,13 which also held true for vitamin C in combination with deferoxamine against Gram-positive cocci, such as S. aureus and S. epidermidis, as well as against Gram-negative bacilli, including E. coli, K. pneumoniae and P. mirabilis.14
Synergistic antibacterial effects could also be observed upon co-administration of vitamin C and quercetin,15 whereas the combination of vitamin C with natural extracts such as pomegranate rind extracts16 and white tea17 resulted in enhanced anti-S. aureus properties of the latter.
Previous studies using combinations of vitamin C with other natural components focused on their antibacterial efficacy. In this study we tested antibacterial efficacy of the combination of vitamin C with L-lysine due to additional benefits of these nutrients in protecting integrity of biological barriers used as entry points for various infection agents.18 The integrity of the endothelial lining of blood vessels, intestines, lung passages, and many other organs largely depends on optimum collagen production.
The study presented here shows bacteriostatic and bactericidal effects of vitamin C and L-lysine used individually and in a combination against A. baumannii and E. coli.
Materials and Methods
Test compounds, test bacterial strains and their growing conditions.
Acinetobacter baumannii Bouvet and Grimont (ATCC® 19606™) and Escherichia coli Migula Castellani and Chalmers (ATCC® 9637™) were chosen as test bacterial strains and cultured in nutrient medium (ATCC, Manassas VA) in 37°C with 5% CO2. Vitamin C and L-lysine were purchased from Sigma (Burlington, MA). LysinC Drink Mix was kindly gifted by Dr. Rath International, Inc. (San Jose, CA). A stock solution (50-100 mg/ml) of each compound (depending on solubility of the substance) was prepared by suspending a test compound (individually or in combination) in 1 x PBS and sterilized by 0.22 µm syringe filtration. All stock solutions were prepared just before the start of the experiment and used immediately.
Evaluation of the bacteriostatic and bactericidal effects of test compounds and their combination against the planktonic form of A. baumannii and E. coli.
Growth inhibition of test strains was tested using a standard macro-dilution method to establish value of bacteriostatic effect.19 Briefly, sterile 3 ml two-position-capped test tubes containing 1 ml nutrient broth with 1 x 106 cfu/ml of the homogenous bacterial suspension were supplemented with the test compounds or their combination. The tubes were then incubated at 37°C with 5% CO2 and growth inhibition as a decrease in the optical density (OD600) was registered after 24 hours of incubation. Control cultures were treated with 1 x PBS.
The bacterial effect of test compounds or their combination was tested on test bacterial strains using standard method.19 The bactericidal values were determined from a broth dilution minimum inhibitory concentration test by sub-culturing the bacterial cell samples removed after 24 hours of incubation, and plated onto nutrient agar plates that did not contain the test agent. The plates were then further incubated at 37°C with 5% CO2 and bacterial re-growth was assessed after 24 hours by counting the colonies.
A bacteriostatic effect was defined as at least 2-log10 cfu/ml reduction, whereas a bactericidal effect was defined as at least 3-log10 cfu/ml decrease from the original inoculum. All experiments were conducted three times independently and each one in three replicates.
Statistical analysis.
All data are presented as means ± SD (n = 3). The Student’s two-tailed t test was used to determine statistically significant differences set at 0.05 levels. Statistical analysis was performed using GraphPad software.
Results and Discussion
The results on Figure 1 show bacteriostatic and bactericidal effects of vitamin C and L-lysine against E. coli and A. baumannii, applied individually and in combination as: vitamin C + L-lysine. In addition, a commercially available composition of vitamin C with L-lysine and citrus bioflavonoids (i.e., LysinC Drink Mix) was also tested.
Neither vitamin C nor L-lysine demonstrated bacteriostatic (when applied at 2.0 mg/ml concentration, respectively), and bactericidal effect (applied at 2.5 mg/ml concentration, respectively), against E. coli and A. baumannii according to “golden standard” definition (bacteriostatic effect defined as 2-log10 cfu/ml and bactericidal effect defined as a 3-log10 cfu/ml decrease). However, the combination of these two compounds with and without citrus bioflavonoids had significant anti-bacterial effects. L-lysine + vitamin C (+/- bioflavonoids) had about 6-7-fold decrease of bacterial growth and about 99.99% bactericidal effects on these two Gram-negative strains, respectively.
These results confirm that vitamin C and L-lysine applied in a combination can significantly enhance each other’s efficacy. This combination can also have possible enhanced antimicrobial benefits through other cellular mechanisms facilitated by these nutrients, since they are also important for preserving integrity of biological membranes as well as antioxidant, and immune enhancing properties, all of which are essential in mounting effective antimicrobial action.
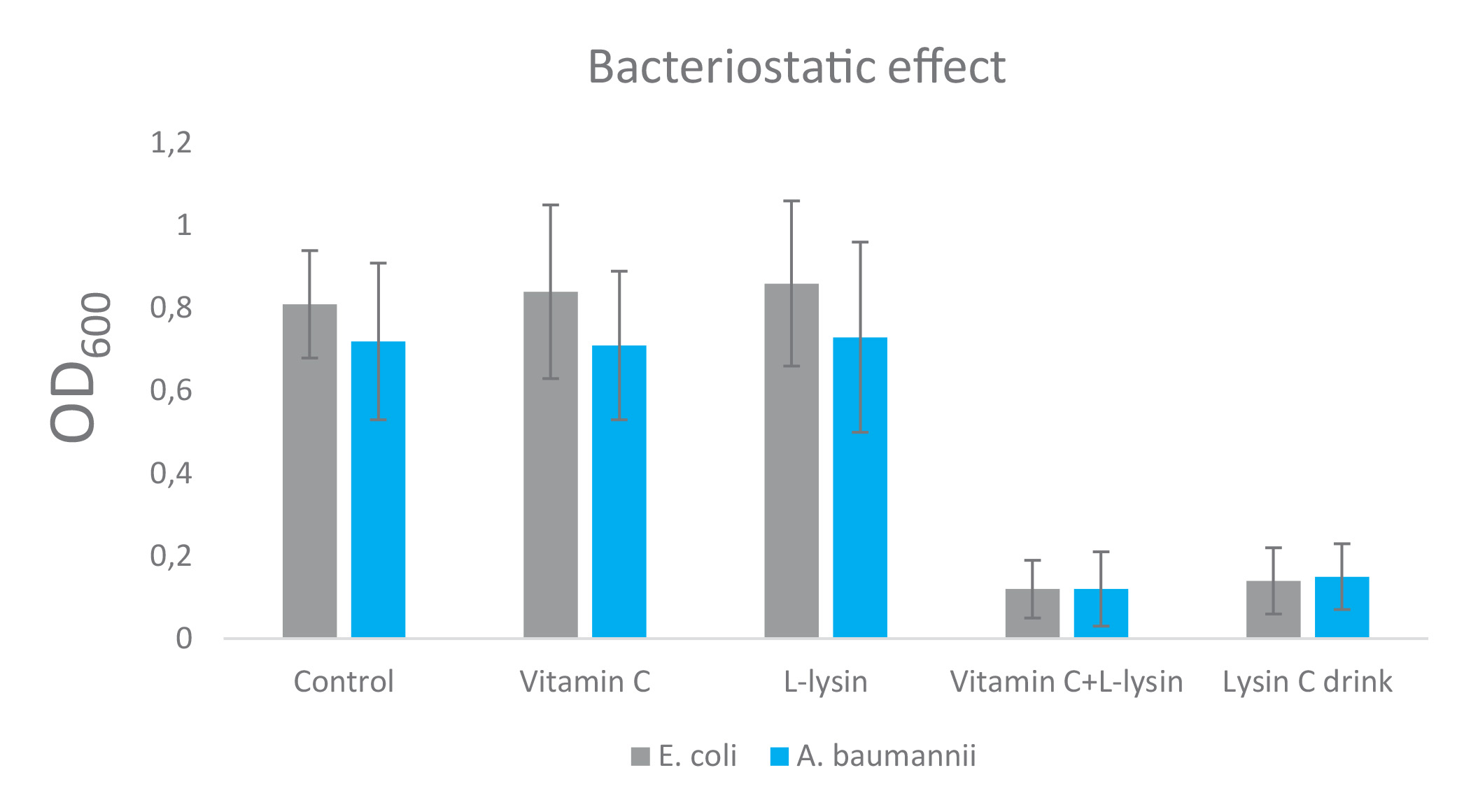
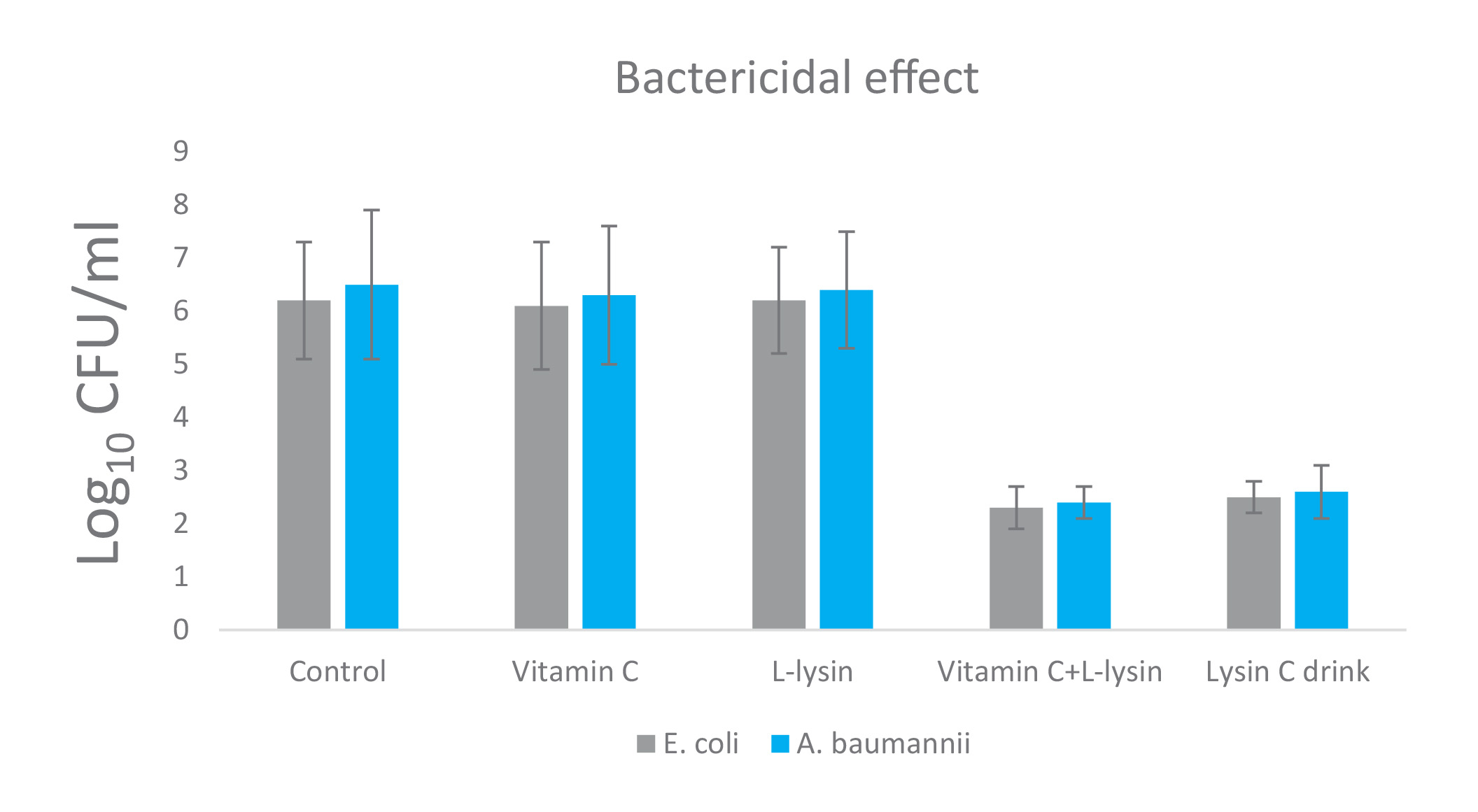
Figure 1. Bacteriostatic and bactericidal effect of Vitamin C, L-lysin, and the mix of these two compounds (1.0 mg/ml each) on two Gram (-) strains: E. coli and A. baumannii for 24 h at 37 °C. Values shown are mean ± standard deviation (n = 4). Bacteriostatic effect was determined by standard macro-dilution assay. Bactericidal was determined from broth macro-dilution tube test by sub-culturing it to agar plates that do not contain the test agent.
Summary: Approximately a 6-7 fold decrease in bacterial growth of Gram (-) strains treated with the mix of Vitamin C and L-lysin, but not with either Vitamin C or L-lysin only, was observed. Approximately a 3.5-4 fold biocidal effect of Gram (-) strains treated with the mix of Vitamin C and L-lysin, but not with either Vitamin C or L-lysin, only was observed.
This result is in line with our earlier work that led to the development of micronutrient combinations with expanded biological targets in bacterial infections20 as well as viral infections21, SARS-CoV-2 and its variants22, HIV-AIDS,23 viral-cancers,24 and others. We have demonstrated that these specific nutrient complexes were effective against multiple steps involved in biological pathways of bacterial and viral replication, and infectivity as well as in curbing cellular mechanisms complementing and promoting infections, i.e., inflammation.
Our results point out that combination of L-lysine with vitamin C should be further explored as an effective measure to simultaneously control and expand cellular mechanisms relevant to managing bacterial infectivity. Its additional benefits include enhanced efficacy at moderate doses of individual nutrients and general safety.
REFERENCES
- Grenni P, Ancona V, Caracciolo AB. 20018. Ecological effects of antibiotics on natural ecosystems: A review. Microchem J. 136: 25-39.
- Alsan M, Klompas M. 2010. An emerging and important pathogen. J Clin Outcomes Manag. 17:363–369.
- Lemos EV, de la Hoz FP, Einarson TR, McGhan WF, Quevedo E, Castañeda C, Kawai K. 2014. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 20:416–423.
- Falagas ME, Rafailidis PI. 2007. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care. 11:134.
- Venkatesh M, Barathi VA, Goh ETL, Anggara R, Fazil M, Ng AJY, et al. 2017. Antimicrobial activity and cell selectivity of synthetic and biosynthetic mrsa. Antimicrob Agents Chemother. 61: e00469-17.
- Švediene J, Novickij V, Žalneraviˇcius R, Raudoniene V, Markovskaja S, Novickij J, Paškeviˇcius A. 2021. Antimicrobial Activity of L-Lysine and Poly-L-Lysine with Pulsed Electric Fields, Appl Sci. 11:2708 https://doi.org/10.3390/app11062708.
- Boissevain CH, Spillane JH., Jr. 1937. A note on the effect of synthetic ascorbic acid (vitamin C) on the growth of the tubercle bacillus. American Review of Tuberculosis. 35:661–662.
- Mousavi S, Bereswill S, Heimesaat MM. 2019. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur J Microbiol Immunol (Bp). 9:73-79 doi: 10.1556/1886.2019.00016.
- Golonka I, Oleksy M, Junka A, Matera-Witkiewicz A, Bartoszewicz M, Musiał W. 2017. Selected Physicochemical and Biological Properties of Ethyl Ascorbic Acid Compared to Ascorbic Acid. Biol Pharm Bull. 40:1199–206.
- Kallio J, Jaakkola M, Mäki M, Kilpeläinen P, Virtanen V. 2012. Vitamin C inhibits staphylococcus aureus growth and enhances the inhibitory effect of quercetin on growth of Escherichia coli in vitro. Planta medica. 78:1824–1830.
- Tajkarimi M, Ibrahim SA. 2011. Antimicrobial activity of ascorbic acid alone or in combination with lactic acid on Escherichia coli O157: H7 in laboratory medium and carrot juice. Food Control. 22:801–4.
- Goswami M, Mangoli SH, Jawali N. 2007. Effects of glutathione and ascorbic acid on streptomycin sensitivity of Escherichia coli. Antimicrob Agents Chemother. 51:1119–1122.
- Hatano T, Tsugawa M, Kusuda M, Taniguchi S, Yoshida T, Shiota S, et al. 2008. Enhancement of antibacterial effects of epigallocatechin gallate, using ascorbic acid. Phytochemistry. 69:3111–3116.
- Van Asbeck B, Marcelis J, Marx J, Struyvenberg A, Van Kats J, Verhoef J. 1983. Inhibition of bacterial multiplication by the iron chelator deferoxamine: potentiating effect of ascorbic acid. Europ J Clin Microbiol. 2:426–431.
- Kallio J, Jaakkola M, Mäki M, Kilpeläinen P, Virtanen V. 2012. Vitamin C inhibits staphylococcus aureus growth and enhances the inhibitory effect of quercetin on growth of Escherichia coli in vitro. Planta medica. 78:1824–1830.
- McCarrell EM, Gould SW, Fielder MD, Kelly AF, El Sankary W, Naughton DP. 2008. Antimicrobial activities of pomegranate rind extracts: enhancement by addition of metal salts and vitamin C. BMC Complemt Alternat Med. 8:64.
- Holloway AC, Gould SW, Fielder MD, Naughton DP, Kelly AF. 2011. Enhancement of antimicrobial activities of whole and sub-fractionated white tea by addition of copper (II) sulphate and vitamin C against Staphylococcus aureus; a mechanistic approach. BMC Complemt Alternat Med. 11:115.
- Galea, I. 2021. The blood–brain barrier in systemic infection and inflammation. Cell Mol Immunol. 18:2489–2501.
- M100-S23. Performance standards for antimicrobial testing; 23rd informational supplement: Clinical and Laboratory Standards Institute; 2013.
- Goc A, Niedzwiecki A, Rath M. 2017. Reciprocal cooperation of phytochemicals and micronutrients against typical and atypical forms of Borrelia sp. J Appl Microbiol. 123:637-650.
- Deryabin PG, Lvov KD, Botikov AG, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. 2008. Effects of a nutrient mixture on infectious properties of a highly pathogenic strain of avian influenza virus A/H5N1. Biofactors. 33(2); 85-97.
- Goc A, Niedzwiecki A, Ivanov V, Ivanova S, Rath M. 2022. Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants. Eur J Microbiol Immunol (Bp). 11:87-94.
- Jariwalla R, Niedzwiecki A, Rath M. 2010. The essentiality of nutritional supplementation in HIV infection and AIDS: Review of clinical studies and results from a Community Health Micronutrient Program. Bioactive Food in Promoting Health: Fruits and vegetables R. Watson, V. Preddy (eds). Elsevier Inc., Chapter 2, pp. 323-342.
- Harakeh S, Diab-Assaf M., Niedzwiecki A., Khalife J., Abu-El-Ardat K., Rath M. 2006. Apoptosis induction by Nutrient Synergy in HTLV-1 positive and negative malignant T-cells. Leukemia Res. 30:869-881.



Figure 1. Bacteriostatic and bactericidal effect of Vitamin C, L-lysin, and the mix of these two compounds (1.0 mg/ml each) on two Gram (-) strains: E. coli and A. baumannii for 24 h at 37 °C. Values shown are mean ± standard deviation (n = 4). Bacteriostatic effect was determined by standard macro-dilution assay. Bactericidal was determined from broth macro-dilution tube test by sub-culturing it to agar plates that do not contain the test agent.
Summary: Approximately a 6-7 fold decrease in bacterial growth of Gram (-) strains treated with the mix of Vitamin C and L-lysin, but not with either Vitamin C or L-lysin only, was observed. Approximately a 3.5-4 fold biocidal effect of Gram (-) strains treated with the mix of Vitamin C and L-lysin, but not with either Vitamin C or L-lysin, only was observed.
REFERENCES
- Grenni P, Ancona V, Caracciolo AB. 20018. Ecological effects of antibiotics on natural ecosystems: A review. Microchem J. 136: 25-39.
- Alsan M, Klompas M. 2010. An emerging and important pathogen. J Clin Outcomes Manag. 17:363–369.
- Lemos EV, de la Hoz FP, Einarson TR, McGhan WF, Quevedo E, Castañeda C, Kawai K. 2014. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 20:416–423.
- Falagas ME, Rafailidis PI. 2007. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care. 11:134.
- Venkatesh M, Barathi VA, Goh ETL, Anggara R, Fazil M, Ng AJY, et al. 2017. Antimicrobial activity and cell selectivity of synthetic and biosynthetic mrsa. Antimicrob Agents Chemother. 61: e00469-17.
- Švediene J, Novickij V, Žalneraviˇcius R, Raudoniene V, Markovskaja S, Novickij J, Paškeviˇcius A. 2021. Antimicrobial Activity of L-Lysine and Poly-L-Lysine with Pulsed Electric Fields, Appl Sci. 11:2708 https://doi.org/10.3390/app11062708.
- Boissevain CH, Spillane JH., Jr. 1937. A note on the effect of synthetic ascorbic acid (vitamin C) on the growth of the tubercle bacillus. American Review of Tuberculosis. 35:661–662.
- Mousavi S, Bereswill S, Heimesaat MM. 2019. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur J Microbiol Immunol (Bp). 9:73-79 doi: 10.1556/1886.2019.00016.
- Golonka I, Oleksy M, Junka A, Matera-Witkiewicz A, Bartoszewicz M, Musiał W. 2017. Selected Physicochemical and Biological Properties of Ethyl Ascorbic Acid Compared to Ascorbic Acid. Biol Pharm Bull. 40:1199–206.
- Kallio J, Jaakkola M, Mäki M, Kilpeläinen P, Virtanen V. 2012. Vitamin C inhibits staphylococcus aureus growth and enhances the inhibitory effect of quercetin on growth of Escherichia coli in vitro. Planta medica. 78:1824–1830.
- Tajkarimi M, Ibrahim SA. 2011. Antimicrobial activity of ascorbic acid alone or in combination with lactic acid on Escherichia coli O157: H7 in laboratory medium and carrot juice. Food Control. 22:801–4.
- Goswami M, Mangoli SH, Jawali N. 2007. Effects of glutathione and ascorbic acid on streptomycin sensitivity of Escherichia coli. Antimicrob Agents Chemother. 51:1119–1122.
- Hatano T, Tsugawa M, Kusuda M, Taniguchi S, Yoshida T, Shiota S, et al. 2008. Enhancement of antibacterial effects of epigallocatechin gallate, using ascorbic acid. Phytochemistry. 69:3111–3116.
- Van Asbeck B, Marcelis J, Marx J, Struyvenberg A, Van Kats J, Verhoef J. 1983. Inhibition of bacterial multiplication by the iron chelator deferoxamine: potentiating effect of ascorbic acid. Europ J Clin Microbiol. 2:426–431.
- Kallio J, Jaakkola M, Mäki M, Kilpeläinen P, Virtanen V. 2012. Vitamin C inhibits staphylococcus aureus growth and enhances the inhibitory effect of quercetin on growth of Escherichia coli in vitro. Planta medica. 78:1824–1830.
- McCarrell EM, Gould SW, Fielder MD, Kelly AF, El Sankary W, Naughton DP. 2008. Antimicrobial activities of pomegranate rind extracts: enhancement by addition of metal salts and vitamin C. BMC Complemt Alternat Med. 8:64.
- Holloway AC, Gould SW, Fielder MD, Naughton DP, Kelly AF. 2011. Enhancement of antimicrobial activities of whole and sub-fractionated white tea by addition of copper (II) sulphate and vitamin C against Staphylococcus aureus; a mechanistic approach. BMC Complemt Alternat Med. 11:115.
- Galea, I. 2021. The blood–brain barrier in systemic infection and inflammation. Cell Mol Immunol. 18:2489–2501.
- M100-S23. Performance standards for antimicrobial testing; 23rd informational supplement: Clinical and Laboratory Standards Institute; 2013.
- Goc A, Niedzwiecki A, Rath M. 2017. Reciprocal cooperation of phytochemicals and micronutrients against typical and atypical forms of Borrelia sp. J Appl Microbiol. 123:637-650.
- Deryabin PG, Lvov KD, Botikov AG, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. 2008. Effects of a nutrient mixture on infectious properties of a highly pathogenic strain of avian influenza virus A/H5N1. Biofactors. 33(2); 85-97.
- Goc A, Niedzwiecki A, Ivanov V, Ivanova S, Rath M. 2022. Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants. Eur J Microbiol Immunol (Bp). 11:87-94.
- Jariwalla R, Niedzwiecki A, Rath M. 2010. The essentiality of nutritional supplementation in HIV infection and AIDS: Review of clinical studies and results from a Community Health Micronutrient Program. Bioactive Food in Promoting Health: Fruits and vegetables R. Watson, V. Preddy (eds). Elsevier Inc., Chapter 2, pp. 323-342.
- Harakeh S, Diab-Assaf M., Niedzwiecki A., Khalife J., Abu-El-Ardat K., Rath M. 2006. Apoptosis induction by Nutrient Synergy in HTLV-1 positive and negative malignant T-cells. Leukemia Res. 30:869-881.

