Health Benefits of Specific Micronutrient Combinations in Diabetes
Currently, diabetes affects more than five hundred million people worldwide and the numbers are projected to increase. Conventional management of diabetes is essentially limited to lowering blood sugar (glucose) levels by means of insulin and other pharmacological drugs. In recent years, certain plant extracts (micronutrients) have been studied for their effects on diabetic metabolism. These studies, however, were largely conducted with individual micronutrients, thereby neglecting the superior synergetic effects of these bioactive molecules in the prevention and therapy of diabetes.
Our study shows that specific combinations of vitamins, minerals and other active plant derived compounds have significant beneficial effects in the simultaneous control of key cellular aspects of sugar (carbohydrate) metabolism and diabetes. These beneficial effects include a significant increase of glucose uptake by skeletal muscle cells of up to 450%. In the presence of insulin, the micronutrient combination tested further enhanced this effect up to 1445% over controls. . The micronutrient combination tested had a comparable efficacy to insulin in facilitating glucose uptake by skeletal muscle cells. Moreover, the same combination of bioactive molecules also stimulated insulin secretion by pancreatic cells by over 230%. In addition, it demonstrated protective effect on glial cells against damage resulting from advanced glycation end products (AGEs), the damaging byproducts of long-term diabetes.
This study confirms that a specific combination of micronutrient formulas can effectively and simultaneously address several cellular mechanisms associated with dysregulated sugar metabolism and diabetic disease. Notably, glucose uptake by human cells could be significantly increased also in the absence of insulin, a finding with relevance to the treatment of type II as well as type I diabetes. These results imply that micronutrients efficacy expands beyond pharmacological treatments by substituting for and supporting insulin in blood sugar metabolism and exercising cellular protection against damage resulting from dysregulated glucose metabolism and diabetes. Thus, properly designed micronutrient compositions can simultaneously address major aspects of diabetic metabolism way beyond the lowering of blood glucose levels and should be considered as a safe and effective measure in both the prevention and management of diabetes.
Introduction
Diabetes is one of the largest epidemics ever to haunt mankind. Currently, more than 6 million people die each year from the cardiovascular complications of diabetes and more than half a billion people worldwide are affected by this disease. 1 These numbers are projected to further increase over the coming decades, reflecting the fact that the metabolic root causes of this pandemic have not been comprehensively addressed at this time.
Diabetes is generally categorized into two main types: Type I Diabetes Mellitus (T1DM) and Type II Diabetes Mellitus (T2DM):
- Type 1 diabetes, about 15% of all cases, occurs primarily in children. The insulin-producing beta-cells of the pancreas are unable to produce adequate amounts of insulin hormone due to genetic defects or damaged beta cells both resulting in severe insulin deficiency. In order to prevent this deadly form of diabetes to develop, these patients remain dependent on regular insulin substitution.
- Type 2 diabetes, about 85% of all cases, generally develops during adulthood and is therefore frequently described as ‘adult-onset diabetes’, however during recent decades an increasing number of children also develops this form of diabetes – largely due to overweight (obesity) and a lack of physical activity. In this case the pancreas cells are able to produce insulin; however, an unhealthy diet and overweight exhaust this hormonal system. As a result, billions of muscle cells and other body cells stop responding properly to insulin. This condition, known as ‘insulin resistance’, causes both, blood sugar and insulin levels, to stay chronically elevated in the blood circulation. Over time, the heavy demands made on the insulin-producing pancreas cells wears them out, and insulin production gradually decrease. 2 3
Effects of diabetes at the cellular level include:
- The ‘sugar coating’ (glycation) of cells and other biological entities of the body by elevated sugar levels (fructose, glucose and others), impairing their regular function, including that of blood cells (oxygen delivery of erythrocytes, immune function of leukocytes).
- Impaired effectiveness of insulin to mediate cellular uptake of sugar molecules and thereby, the decrease of blood sugar levels. The efficacy of insulin to transport sugar molecules from the bloodstream into the cells is dependent on a cellular enzyme (Akt), which has to be energized (phosphorylated) in order to promote glucose transport inside the cells. In diabetes this energizing process is impaired. 4 5
- The formation of complex sugar structures, advanced glycation end products (AGEs) that are deposited inside the blood vessel walls and other organs and can significantly impair normal blood circulation and other organ functions.
- Free radical production and oxidative modification of lipids, proteins, DNA, and other molecules is another cellular mechanism associated with diabetes, a fact that has important implications for the protective use of antioxidants.
- Inflammation is yet another cellular mechanism typically associated with dysregulated sugar metabolism and diabetes.
- A weakening of the principle stability of body tissue by impairing the production and function of collagen and other molecules of the extracellular matrix. This effect is due to a molecular similarity between sugar (glucose) and vitamin C (ascorbic acid) molecules and the fact that elevated sugar levels block the regular uptake of vitamin C in human body cells. Since optimum intracellular amounts of vitamin C is also essential for the optimum production and function of collagen, diabetes reduces body tissue strength in general. 6
The benefits of certain vitamins and other micronutrients in diabetes have been reported by various investigators. For example, arginine and other amino acids were shown to synergize insulin secretion and insulin sensitivity. 7 B-vitamins, such as thiamine, have improved complications of diabetes mellitus. 8 9 Polyphenols have been shown to improve glucose metabolism, independently of insulin, as well as to aid in the prevention of protein glycation, a factor in hemoglobin damage. 10 11 Components in cinnamon extract (catechin, epicatechin, procyanidin B2 and phenol polymers) are known to block AGE products, minimizing the deleterious effects by these intermediaries on the vasculature. 12 Numerous other dietary approaches have been applied to reduce hyperglycemia and insulin resistance 13 14 and modify cardiovascular risk factors in diabetes. 15 16 However, most of these studies have been conducted with individual ingredients, thereby leaving the significant synergistic effects of specific combinations of micronutrients largely unexplored. In our study we tested how different nutritional formulas and their combinations affect cellular metabolic processes that can result in lowering glucose levels and protect against the multitude of mechanisms of cellular damage associated with diabetes.
Materials and Methods
Cell Lines
IMG cell line (microglial cells derived from the brains of adult mice) was purchased from Kerafast. L6 cell line (rat myoblast /skeletal muscle cells) and Beta- TC-6 cell line (insulin secreting cell line derived from a pancreatic tumor (insulinoma) were purchased from ATCC.
Assay kits and Reagents
The following assays were used in the study: Glucose Uptake-Glo™ Assay kit from Promega; Mouse Insulin ELISA Kit from Biovision; Alamar Blue Cell Viability reagent from ThermoFisher; AGE: Glycated Bovine Serum Albumin (BSA) from Abcam. All other chemicals were from Sigma and ATCC. The test formulations: Basic formula for sugar metabolism, Mineral formula and Vitamin D3-K2 formula were from Dr. Rath Health Programs, Heerlen NL.
Methods
Sample preparation
All cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% Penicillin-Streptomycin.
Test nutrient formulations were solubilized as follows: Basic Formula and Mineral Formula were solubilized in 0.1N Hydrochloric Acid according to Pharmacopeia protocols. Briefly, the tablets were crushed and shaken overnight at 37°C in 0.1N HCl. Solution was filter sterilized and frozen in aliquots. D3-K2 Formula was solubilized in 100% DMSO and frozen in aliquots.
It was filter sterilized after dilution with media before adding to cells.
Cell Protection from Glycation
IMG cells were seeded and grown to confluency in clear 96 well plates. They were treated with nutrient mixes together with 1mg/ml AGE in DMEM with 1% FBS. After 24 hours, cells were washed with Phosphate-Buffered Saline (PBS) and Alamar Blue assay was performed to test cells viability. Each treatment was subjected to 6 repetitions.
Glucose Uptake Assay
L6 cells were seeded and grown to confluency in white 96 well plates. After treating with various nutrients in the presence 0.1 nM insulin or without insulin for 24 hours, cells were starved by incubating in DMEM without glucose and serum for 1 hour. After that, the Glucose Uptake Assay kit was used according to manufacturer instructions. Luminescence was measured by a Tecan luminometer. Four repetitions were performed for each treatment when testing individual formulations and three repetitions when testing a micronutrient mix.
Insulin ELISA
Beta TC-6 pancreatic cells were seeded and grown to confluency in 96-well plates. They were treated with nutrient formulas for 48 hours. Cell supernatant was centrifuged to remove sediments and the resulting supernatant was assayed using the Insulin ELISA kit according to manufacturer instructions. Three repetitions were performed for each treatment.
Results
Description of test formulas
Table 1. Compositions of micronutrient complexes tested in the study
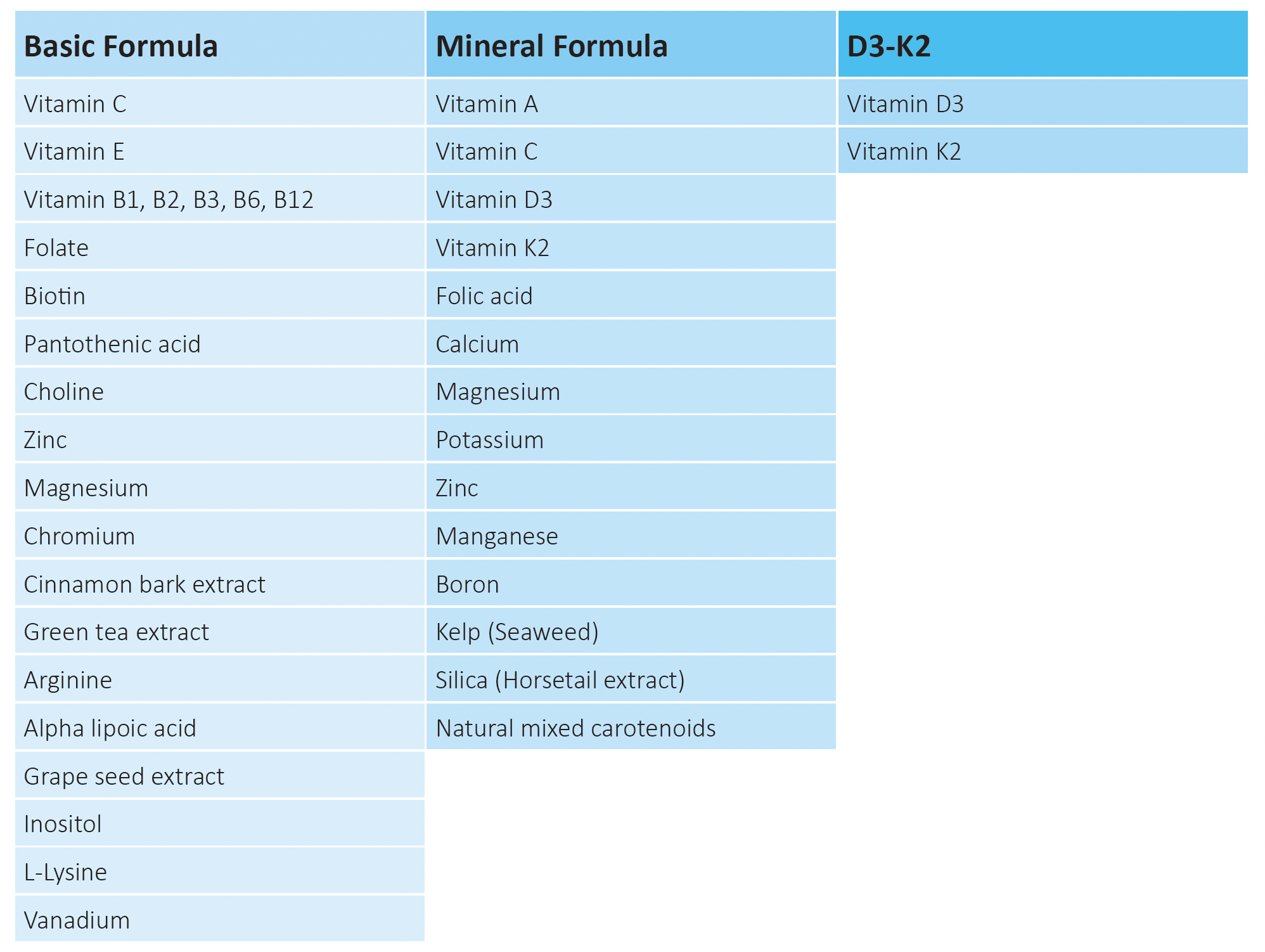
Following three micronutrient compositions were evaluated in the study: Basic Formula containing vitamins with other synergistically acting natural compounds supporting healthy sugar metabolism, Mineral Formula providing various minerals, trace elements and vitamins and a combination of Vitamin D3 and K2 Formula.
Effects of micronutrient complexes and their combinations on glucose uptake by skeletal muscle cells
The effects of Basic Formula, Mineral Formula and D3-K2 Formula on glucose uptake by skeletal muscle cells were evaluated in the absence and presence of insulin as presented on Figure 1 A, B. These micronutrient formulations were applied individually and in a combination. In the absence of insulin (Fig 1A) all test formulas applied individually were effective in increasing glucose uptake by skeletal muscle cells, but to a different degree. The highest efficacy in increasing glucose uptake by 235% was observed with Basic Formula, followed by Mineral Formula with 159% increase and D3-K2 Formula with 37% glucose increase compared to control. The combination of these three formulas significantly exceeded the efficacy of individual formulations by increasing glucose uptake by skeletal muscle cells by 450% compared to control.
In order to see whether these micronutrient formulations retain their efficacy also in the presence of insulin, we evaluated cellular glucose uptake with insulin applied at the concentration corresponding to its average level in a human blood (0.1 nM). The results on Figure 1B show that all test formulas could further enhance glucose uptake by skeletal muscle cells compared to the effect of insulin alone, with a maximum efficacy obtained when all formulas were applied together.
Insulin alone increased glucose uptake by human skeletal muscle cells by 404% compared to control. A further significant increase was observed when insulin was compared with the micronutrient formulas: Insulin combination with the Basic formula increased cellular glucose uptake by 670% an increase of about 266% over insulin alone. The Mineral formula and D3-K2 Formula applied together with insulin increased glucose uptake by 539% and 445%, respectively, compared to control. The combination of all three formulas had the highest stimulatory effect by increasing glucose intake by the cells by 1,445% over control. This result signified a more than 5-time increase over the effect of insulin alone.
Comparison of the efficacy of micronutrient and insulin on glucose uptake by skeletal muscle cells
We investigated the stimulatory effect of micronutrients on glucose uptake by human skeletal muscle cells in comparison to the effects of insulin. Insulin was applied at concentrations of 1 nM that resembles its postprandial level measured at 2 hours after a meal, at 0.1 nM corresponding to insulin levels in the presence of average blood sugar levels, and 0.01nM.
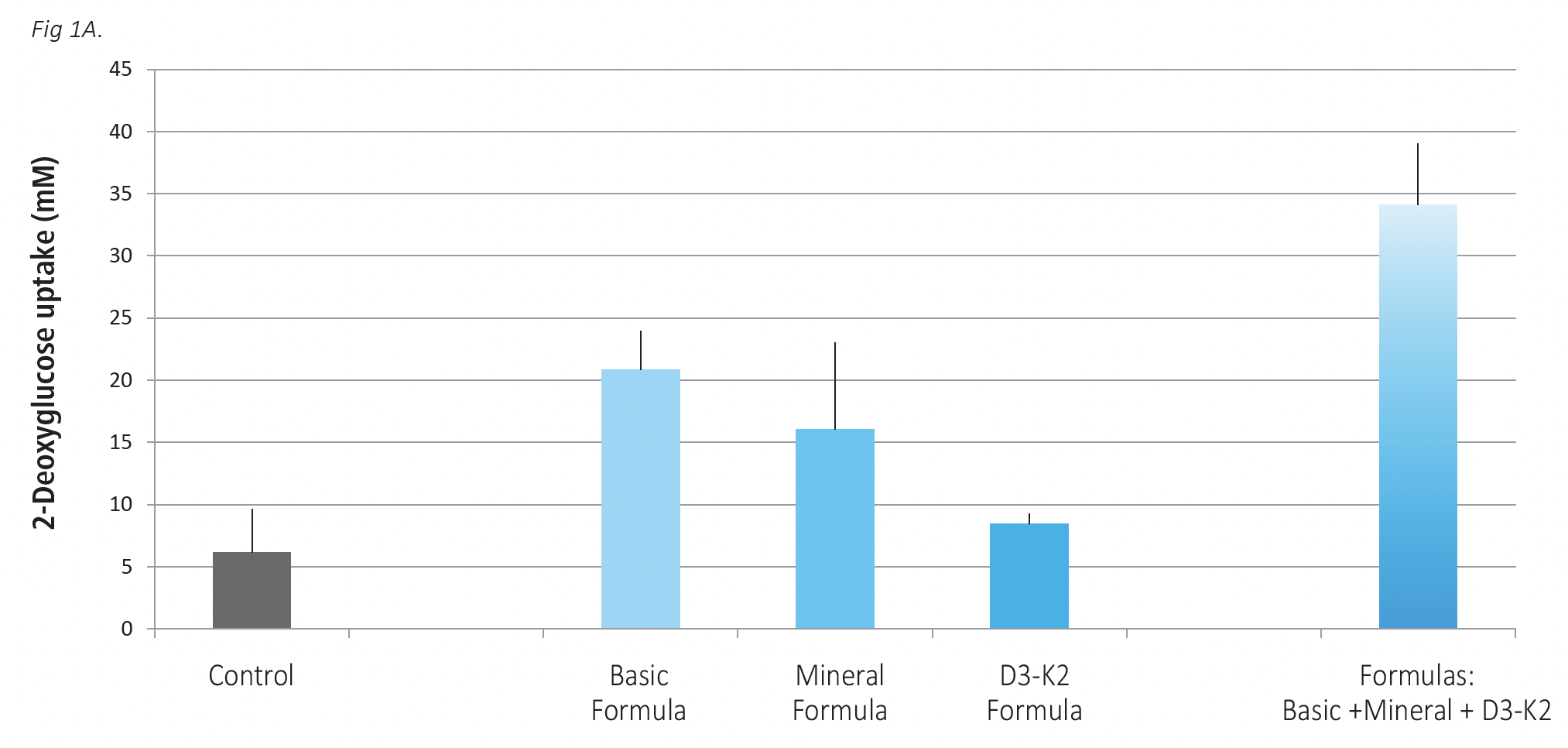
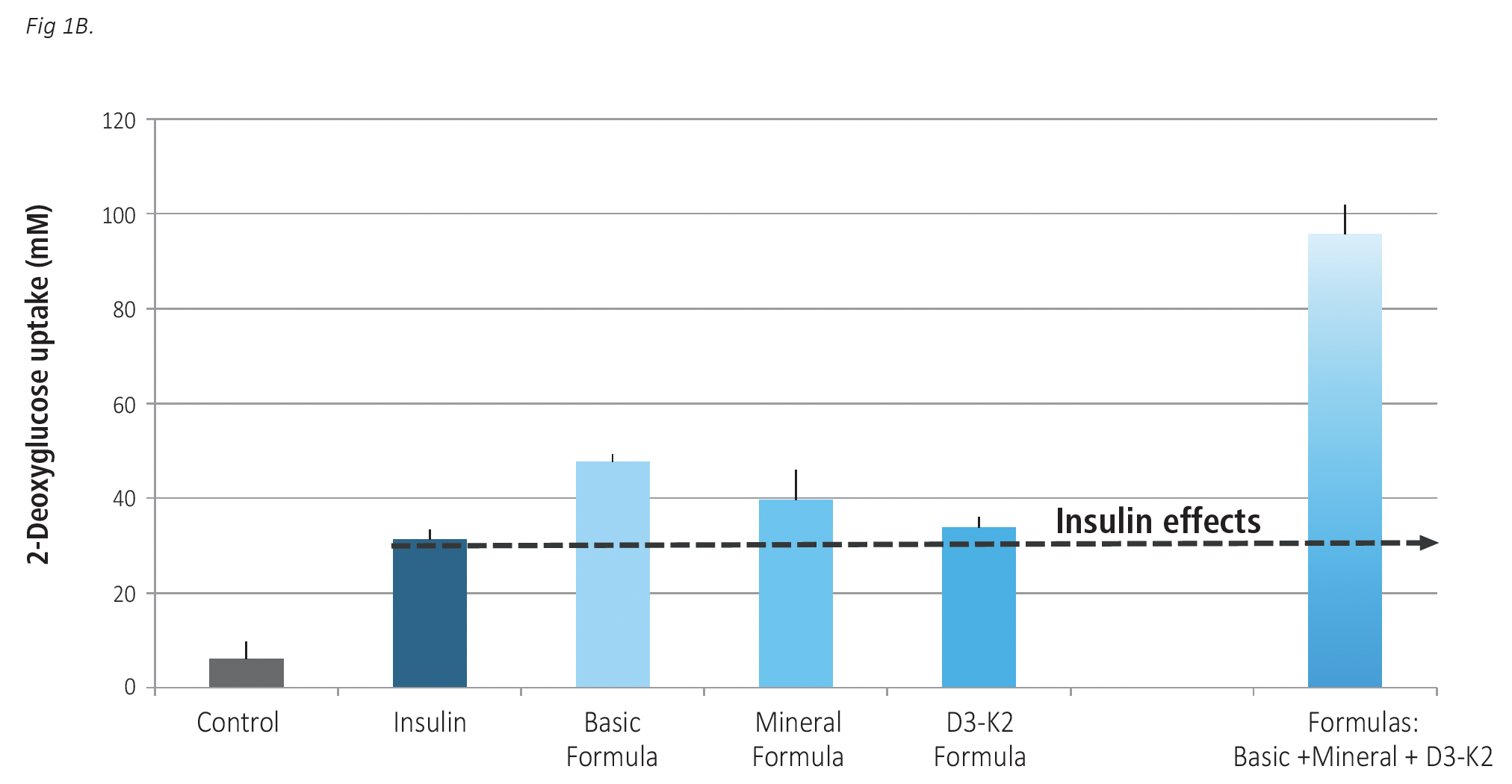
Fig 1 A,B. The effects of Basic Formula, Mineral Formula and D3-K2 Formula applied individually and in a combination on glucose uptake by skeletal muscle cells. Cells were exposed to test formulations for 24 hours, with Basic Formula applied at 16.9 mcg/ml concentration, Mineral formula at 22.5 mcg/ml and D3-K2 Formula at 0.83 ng/m. The results are expressed as average values +/-SD, with individual formulas tested in 4 repetitions and their mix in 3 repetitions. Fig 1 A – tests without insulin; Fig 1B, test in the presence of 0.1 nM insulin.
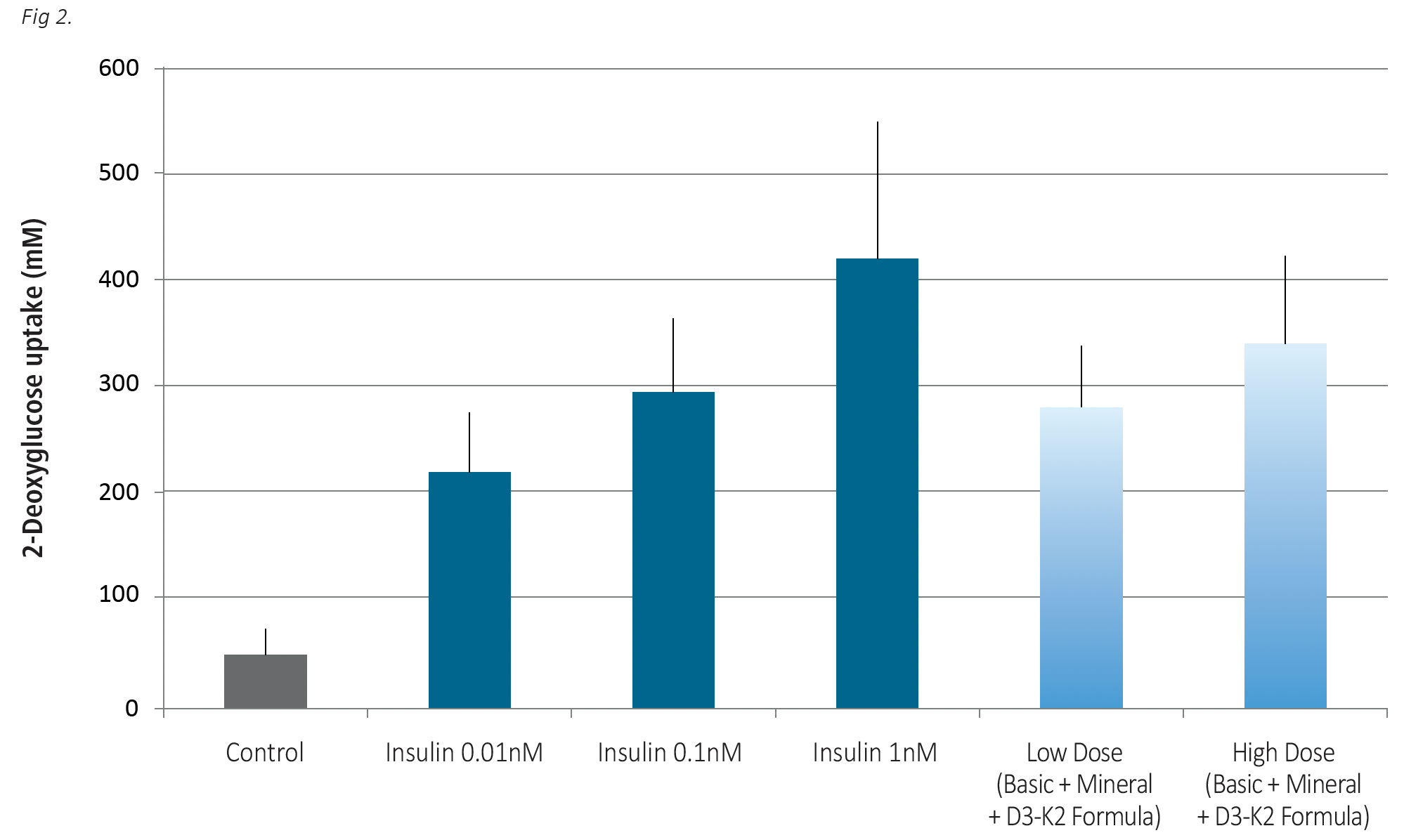 Fig. 2. Effects of the combination of the tested formulations administered at different concentrations on glucose uptake by skeletal muscle cells when compared to the effects of insulin. Cells were exposed to low (9.86 mcg/ml) and high (49.4 mcg/ml) concentrations of the combined formulations and to insulin doses at 0.01 nM, 0.1 nM and 1.0 nM) for 24 hrs.
Fig. 2. Effects of the combination of the tested formulations administered at different concentrations on glucose uptake by skeletal muscle cells when compared to the effects of insulin. Cells were exposed to low (9.86 mcg/ml) and high (49.4 mcg/ml) concentrations of the combined formulations and to insulin doses at 0.01 nM, 0.1 nM and 1.0 nM) for 24 hrs.
As shown in Fig. 2 the combination of three formulas applied at a lower concentration (9.86 mcg/ml) had a stimulatory effect on glucose uptake comparable to that in the presence of average insulin level. With below normal insulin level (0.01nM) glucose uptake by the cells equaled 219mM. The combination of the three Formulas applied both at the low and high dose surpassed the glucose uptake in the presence of 0.01nM insulin, and were assessed at 281mM and 340 mM, respectively.
The insulin alone applied at its high (postprandial) concentration was more effective than high dose of micronutrients in facilitating glucose uptake by the cells (419 mM vs 340 mM). Further studies should assess, whether glucose uptake by skeletal muscle cells can be further enhanced to match that of postprandial levels by further increasing the micronutrient dosage.
Effects of individual micronutrient formulas and their combination in protecting cells against AGE induced damage
Advanced glycation end products (AGE) represent harmful by-products of diabetic metabolism on biological structures like proteins. Since nervous tissue can be significantly damaged by a long-term exposure to high blood sugar, we tested whether individual micronutrient formulations and their combination can protect glial nerve cells against damage from AGE proteins.
The results in Figure 3 show that in the presence of AGE glycated proteins the glial cells die. In contrast, the presence of the tested micronutrient combinations significantly increased the survival rate of nerve cells exposed to harmful AGEs. Among individual formulas the highest protective effect was observed in the presence of the D3-K2 Formula, resulting in the survival of 40% of the cells. When all test formulas were applied simultaneously, 80% of the cells resisted AGE damage and survived.
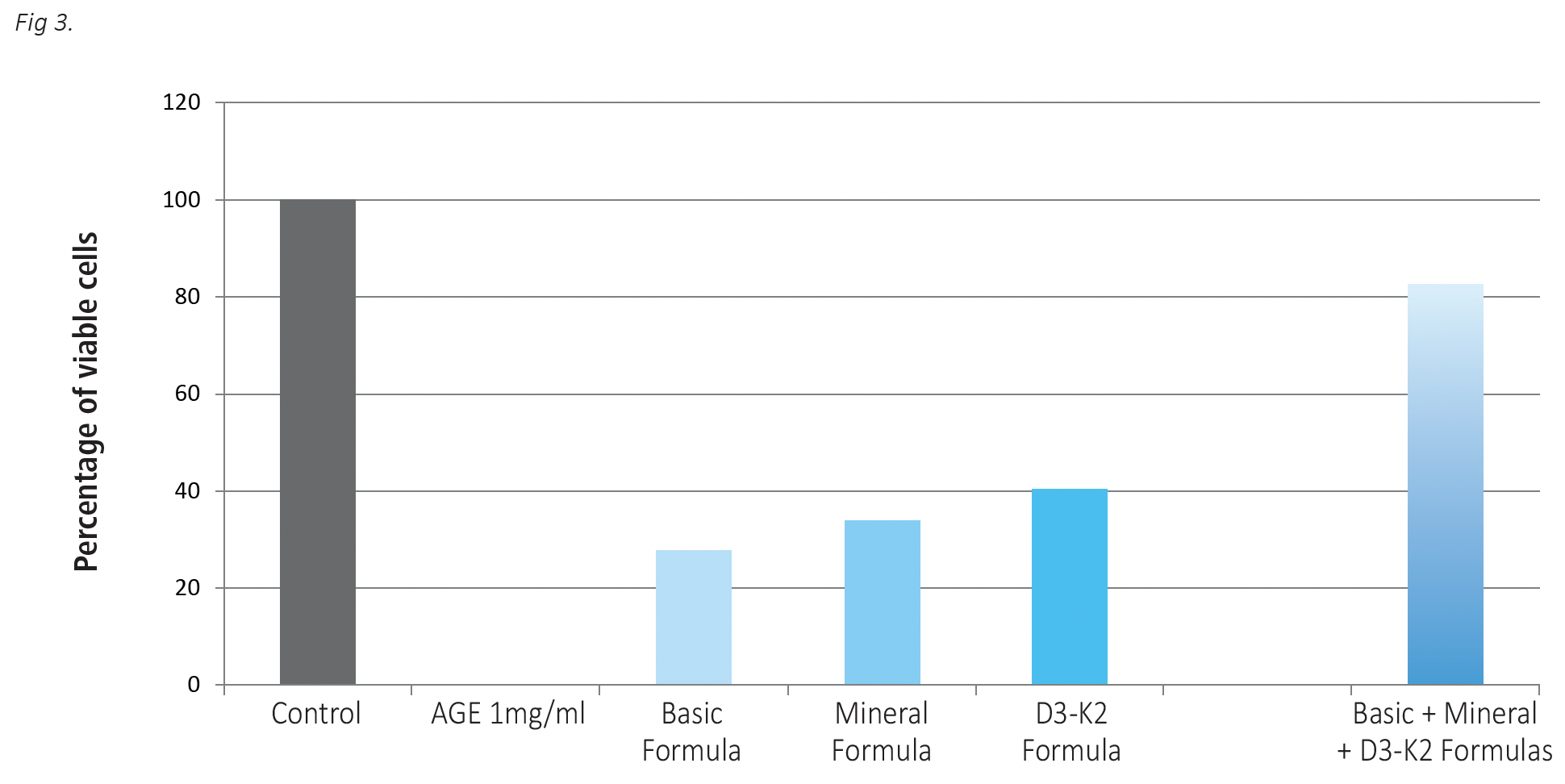
Fig 3. The effects of test micronutrient formulations used individually and in a combination on protection of glial cells against damage by high glycation products (AGE). Glial cells were incubated in the presence of AGE at 1mg/ml without and with Basic Formula at 4.23 mcg/ml, Mineral Formula at 5.63 mcg/ml and D3-K2 Formula at 0.21 ng/ml for 24 hours. Cell viability was evaluated using Alamar Blue assay (See Material and Methods). The results are expressed as average values +/-SD, obtained for 6 repetitions of each treatment.
Effects of micronutrient combination on insulin secretion by pancreatic cells
The results on Fig. 4 show that pancreatic cells incubated in the presence of increasing doses of a combination of the formulas can significantly increase insulin secretion. At the higher dose of micronutrients used in this study (78.8mcg/ml) insulin secretion increased by 232% compared to control.
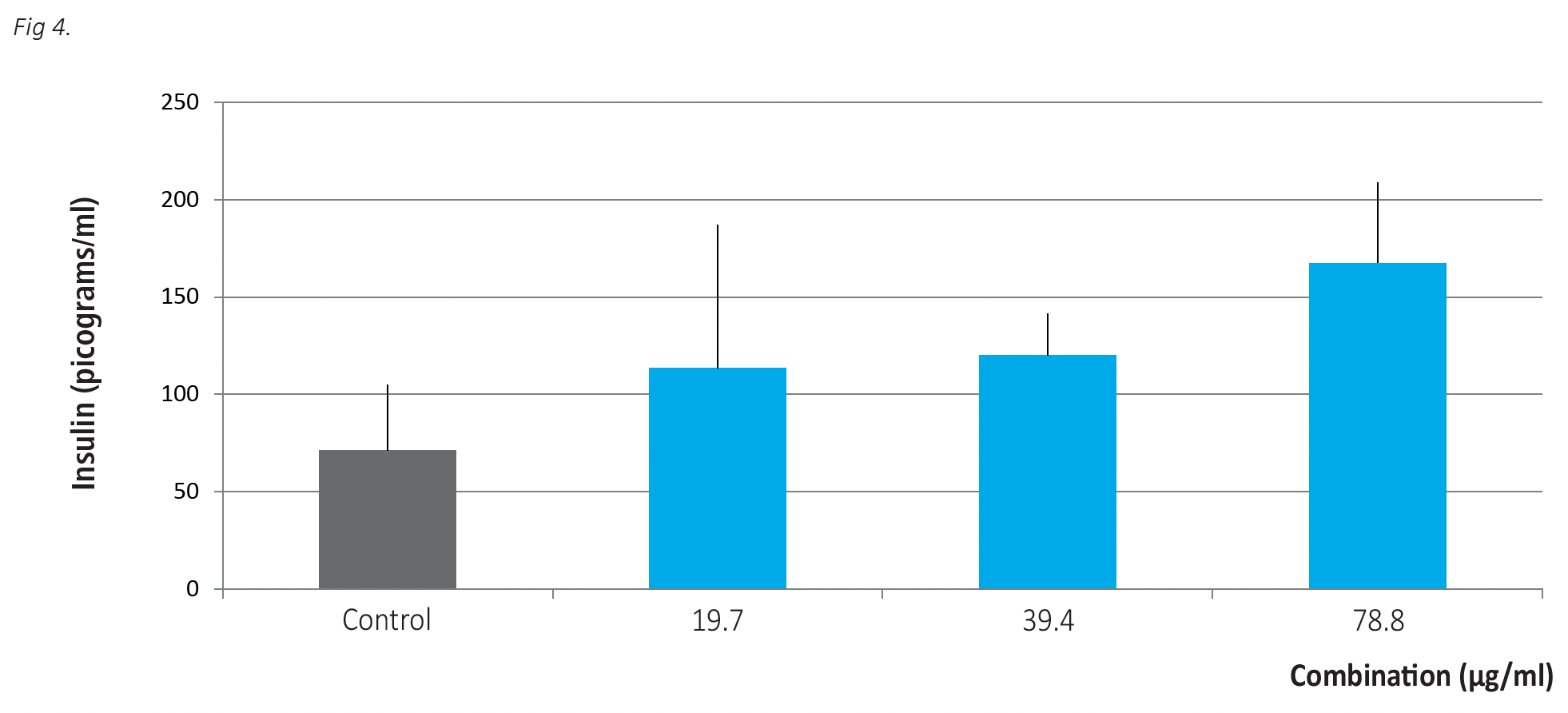 Fig 4. The effects of the combination of individual formulas on insulin secretion by human pancreatic cells after 24 hours incubation. Insulin was detected by ELISA as described in Materials and Methods. The results are expressed as average values +/-SD, obtained for three repetitions of each treatment.
Fig 4. The effects of the combination of individual formulas on insulin secretion by human pancreatic cells after 24 hours incubation. Insulin was detected by ELISA as described in Materials and Methods. The results are expressed as average values +/-SD, obtained for three repetitions of each treatment.
Discussion
Micronutrients in normalizing glucose metabolism: Optimum cellular glucose uptake and utilization facilitates bioenergy production and heathy metabolism. Our results scientifically support the efficacy of three different micronutrient formulations in critical cellular processes essential in normalizing blood glucose levels, promoting insulin secretion and protecting human cells from the damaging effects of diabetic metabolism.
The efficacy of individual test formulas differed, but their combination had superior effects on increasing glucose uptake by skeletal muscle cells both in the absence and presence of insulin and supporting insulin secretion by the pancreatic cells. Among individual formulas, the highest efficacy in increasing cellular glucose uptake was observed with Basic Formula. This formula contains several ingredients such as cinnamon extract, B- vitamins, vitamin C, chromium, vanadium among others that showed to be effective in normalizing blood glucose levels in clinical settings. Vitamin C is important since hyperglycemia deprives cells of vitamin C, causing collagen impairment and pathological changes in the cardiovascular system as well as other pathologies 6.
A Mineral Formula containing minerals, such as magnesium, calcium, manganese, zinc and iodine in addition to vitamin C, A, E, D3 and K2 was also effective in increasing glucose uptake by the skeletal muscle cells both in the presence and absence of insulin. Deficiencies of minerals and trace elements play important roles in the pathogenesis and progression of type 2 diabetes and the mode of action of a number of trace elements is altered in Type 2 diabetes 17. Magnesium reduces insulin resistance in people at risk for Type 2 diabetes 18 19 and various clinical trials found benefits from its supplementation in diabetics 20. Zinc, chromium and selenium have been shown to potentiate insulin action 21. Zinc also acts as insulin mimetic, activating key molecules implicated in cell signaling to maintain glucose homeostasis in mouse and human skeletal muscle cells 22.
The findings reported here corroborate with earlier investigations at our institute that established the effect of micronutrients on cytokines and other intracellular signaling pathways in thwarting the damaging effects of diabetic metabolism. (5). In these studies, specific micronutrient combinations were able to more than double the phosphorylation of the intracellular AKT protein, a key step in the cellular uptake of glucose molecules and their removal from the blood stream.
A significant result of this study was the synergetic effect of the three formulas tested. This micronutrient synergy rivaled the efficacy of insulin in regulating glucose uptake by human body cells. This role of micronutrients in enhancing cellular uptake of glucose was complemented by a significant stimulation of insulin production in pancreatic cells.
Micronutrients in preventing high sugar related cellular pathology: High blood sugar levels lead to various pathological changes to the body tissues and organs resulting from damage to biological molecules cross-linked as a result of the glycation process. Altered structure and function of proteins such as collagen increases the risk of atherosclerosis, kidney damage, retinopathy among many others. One of the most debilitating effects in the course of diabetes is the deposition of advanced glycation end products (AGEs) in the vicinity of nerve cells, eventually impairing movement, sensation and other neuronal functions, commonly known as diabetic polyneuropathy. Research in this area has mainly focused directly on the neuronal cells, however much less is known about the diabetic impairment of glial cells that surround the nerve cells. Glial cells are vital for neuronal functioning by providing support and protection, cleaning up debris, and forming myelin. Because of this protective role of glial cells for neurons, we were interested whether the very same micronutrient combinations that were effective in improving glucose metabolism also have a protective effect against AGE-induced cell damage.
We observed that glial cells that were exposed to increasing concentrations of AGEs in the absence of micronutrients change their morphology and die. However, the presence of specific micronutrient combinations had a significant protective effect against AGEs, resulting in the survival of 80% of cells. Interestingly among individual formulas, the highest protective effect against cell damage was observed in the presence of the formula containing vitamins D and K2. Vitamin D deficiency is known to be prevalent in patients with type 2 diabetes, particularly those with symptoms of neuropathy, and low serum vitamin D levels are an independent predictor of diabetic polyneuropathy development 23 24.
Vitamin K2 is important in the synthesis of sphingolipids present in high concentrations in brain cell membranes and is involved l in brain cells development and survival 25. Therapeutic benefits of vitamin K2 in reducing neuropathy symptoms, pain and other have been demonstrated in patients with Type 2 diabetes 26. A combination of both vitamins may further enhance health benefits by simultaneously affecting various cellular mechanisms and through their synergistic effect.
CONCLUSION
Our results imply that after 100 years of relying on insulin and other glucose-lowering antidiabetic drugs in the management of diabetes, science has identified new effective and safe ways to be used as adjuncts and/or alternatives. The potential advantage of this new approach derives from the fact that it not only contributes to blood sugar decrease but – simultaneously – benefits all key aspects of diabetic metabolism, including facilitating glucose transport inside the muscle cells, enhanced insulin secretion by pancreatic cells and protection against damaging AGE formation.
Taking into account that the micronutrient combinations tested here have a beneficial effect on all key pathologies associated with diabetes, this natural and safe new approach should be validated in clinical settings in all forms of this disease, diabetes type I and type II as well as gestational diabetes.
Since the comprehensive biological effects of the micronutrient combinations described here are due to their regulatory role on cellular metabolism, positive results from clinical studies with these micronutrients in diabetic patients would – inevitably – pave the way to an effective and safe preventive approach to diabetes and could effectively curtail this pandemic.
REFERENCES
- https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html
- Cryer Wilson JD, Foster DW. Glucose homeostasis and hypoglycaemia. In William’s Textbook of Endocrinology., Eds. Philadelphia, Pa., W.B. Saunders Company, 1992, p.1223–1253
- Gerich JE, Langlois M, Noacco C, Karam JH, Forsham Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182(4108):171-173. doi:10.1126/science.182.4108.171
- Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt Diabetes. 2005; 54(8):2360-2364. doi:10.2337/diabetes.54.8.2360
- Chatterjee M, Ivanov V, Niedzwiecki A, Rath M: Micronutrient complexes support glucose metabolism in skeletal muscle J Cellular Medicine and Natural. Health 2019. May. (Available at: https://jcmnh.org/index.php/2019/05/13/micronutrient-complexes-support-glucose- metabolism-in-skeletal-muscle-cells)
- Rath Why Animals Don’t Get Heart Attacks … But People Do! Dr. Rath Health Foundation July 2018
- Miczke A, Suliburska J, Pupek-Musialik D, et al. Effect of L-arginine supplementation on insulin resistance and serum adiponectin concentration in rats with fat diet. Int J Clin Exp Med. 2015;8(7):10358-10366. 2015 Jul 15.
- González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, Ramírez-Ramírez V and Ramos-Zavala MG: Effect of thiamine administration on metabolic profile, cytokines and inflammatory markers in drug-naïve patients with type 2 Eur J Nutr. 2011; 50(2): 145-149. doi:10.1007/ s00394-010-0123-x
- Arora S, Lidor A, Abularrage CJ, et Thiamine (vitamin B1) improves endothelium-dependent vasodilatation in the presence of hyper glycemia. Ann Vasc Surg. 2006; 20(5): 653-658.
- Zygmunt K, Flaubert B, MacNeil J and Tsiani E. Naringenin, a citrus bioflavonoid, increases muscle cell glucose uptake via Biochem Biophys Res Commun. 2010; 398: 178-183. doi:10.1016/j.bbrc.2010.06.048
- Urios P, Grigorova-Borsos AM and Sternberg Flavonoids inhibit the formation of the cross-linking AGE pentosidine in collagen incubated with glucose, according to their structure. Eur J Nutr. 2007; 46: 139-146.
- Peng X, Cheng KW, Ma J, Chen B, Ho CT, Lo C, Chen F and Wang M: Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation J Agric Food Chem. 2008;56(6): 1907- 1911. doi:10.1021/jf073065v
- Suwannaphet W, Meeprom A, Yibchok-Anun S and Adisakwattana Preventive effect of grape seed extract against high-fructose diet-induced insulin resistance and oxidative stress in rats. Food Chem Toxicol. 2010; 48(7): 1853-1857. doi:10.1016/j.fct.2010.04.021
- Liu J, Sun H, Duan W, Mu D and Zhang L. Maslinic acid reduces blood glucose in KK-Ay Biol Pharm Bull. 2007; 30(11): 2075-2078. doi:10.1248/bpb.30.2075
- Yi X and Maeda N: Alpha-lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006; 55(8): 2238-2244. doi:10.2337/db06-0251
- Cha J, Ivanov V, Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M. Nutritional improvement of metabolic syndrome parameters in immature fructose-fed wild type mice. Mol Med Rep 2011;4(6):1053-1059. doi:10.3892/mmr.2011.562
- Sujatha P. Trace Elements in Diabetes Mellitus. Clin. Diagn. Res. 2013;7:1863–1865. doi:10.7860/JCDR/2013/5464.3335
- Fung, T., Manson, J. E., Solomon, C. G., Liu, S., Willett, W. C., & Hu, F. B. The association between magnesium intake and fasting insulin concentration in healthy middle-aged women. J Am Coll of Nutr. 2003; 22(6), 533–538. doi:10.1080/07315724.2003.10719332
- Barbagallo , Dominguez L.-J. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch. Biochem. Biophys. 2007;458(1):40–47. doi: 10.1016/j.abb.2006.05.007
- Yao X, Liu R, Li X, Li Y, Zhang Z, Huang S, Ge Y, Chen X, Yang X. Zinc, selenium and chromium co-supplementation improves insulin resistance by preventing hepatic endoplasmic reticulum stress in diet-induced gestational diabetes rats. Nutr. Biochem. 2021; 96: 108810. doi:10.1016/j.jnutbio.2021.108810
- Norouzi S, Adulcikas J, Sohal SS, Myers S , Zinc stimulates glucose oxidation and glycemic control by modulating the insulin signaling pathway in human and mouse skeletal muscle cell lines. PLoS ONE. 2018; 13(1): e0191727. https://doi.org/10.1371/journal.pone.0191727
- Yammine K, Wehbe R, Assi C. A systematic review on the efficacy of vitamin D supplementation on diabetic peripheral neuropathy. Clin Nutr. 2020; 39(10): 2970-2974. doi:10.1016/j.clnu.2020.01.022
- Ahmadieh H, Azar ST, Lakkis N, Arabi A. Hypovitaminsis d in patients with type 2 diabetes mellitus: a relation to disease control and ISRN Endocrinol. 2013;641098. doi:10.1155/2013/641098
- Fournet M, Bonté F, Desmoulière A: Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging Dis. 2018;9(5):880-900. Published 2018 Oct 1. doi: 10.14336/AD.2017.1121
- 25. Shu-Hsiang Huang, Sheng-Ting Fang and Yi-Cheng Chen. Molecular Mechanism of Vitamin K2 Protection against Amyloid-β-Induced Cytotoxicity. Biomolecules. 2021; 11(3), 423. Published 2021 Mar 13. https://doi.org/10.3390/biom11030423
- Mehta DS, Dound YA, Jadhav SS, Bhave AA, Devale M, Vaidya A novel potential role of Vitamin K2-7 in relieving peripheral neuropathy. J Pharmacol Pharmacother. 2018;9:180-5

Fig 1 A,B. The effects of Basic Formula, Mineral Formula and D3-K2 Formula applied individually and in a combination on glucose uptake by skeletal muscle cells. Cells were exposed to test formulations for 24 hours, with Basic Formula applied at 16.9 mcg/ml concentration, Mineral formula at 22.5 mcg/ml and D3-K2 Formula at 0.83 ng/m. The results are expressed as average values +/-SD, with individual formulas tested in 4 repetitions and their mix in 3 repetitions. Fig 1 A – tests without insulin; Fig 1B, test in the presence of 0.1 nM insulin.

Fig 1 A,B. The effects of Basic Formula, Mineral Formula and D3-K2 Formula applied individually and in a combination on glucose uptake by skeletal muscle cells. Cells were exposed to test formulations for 24 hours, with Basic Formula applied at 16.9 mcg/ml concentration, Mineral formula at 22.5 mcg/ml and D3-K2 Formula at 0.83 ng/m. The results are expressed as average values +/-SD, with individual formulas tested in 4 repetitions and their mix in 3 repetitions. Fig 1 A – tests without insulin; Fig 1B, test in the presence of 0.1 nM insulin.

Fig. 2. Effects of the combination of the tested formulations administered at different concentrations on glucose uptake by skeletal muscle cells when compared to the effects of insulin. Cells were exposed to low (9.86 mcg/ml) and high (49.4 mcg/ml) concentrations of the combined formulations and to insulin doses at 0.01 nM, 0.1 nM and 1.0 nM) for 24 hrs.

Fig 3. The effects of test micronutrient formulations used individually and in a combination on protection of glial cells against damage by high glycation products (AGE). Glial cells were incubated in the presence of AGE at 1mg/ml without and with Basic Formula at 4.23 mcg/ml, Mineral Formula at 5.63 mcg/ml and D3-K2 Formula at 0.21 ng/ml for 24 hours. Cell viability was evaluated using Alamar Blue assay (See Material and Methods). The results are expressed as average values +/-SD, obtained for 6 repetitions of each treatment.
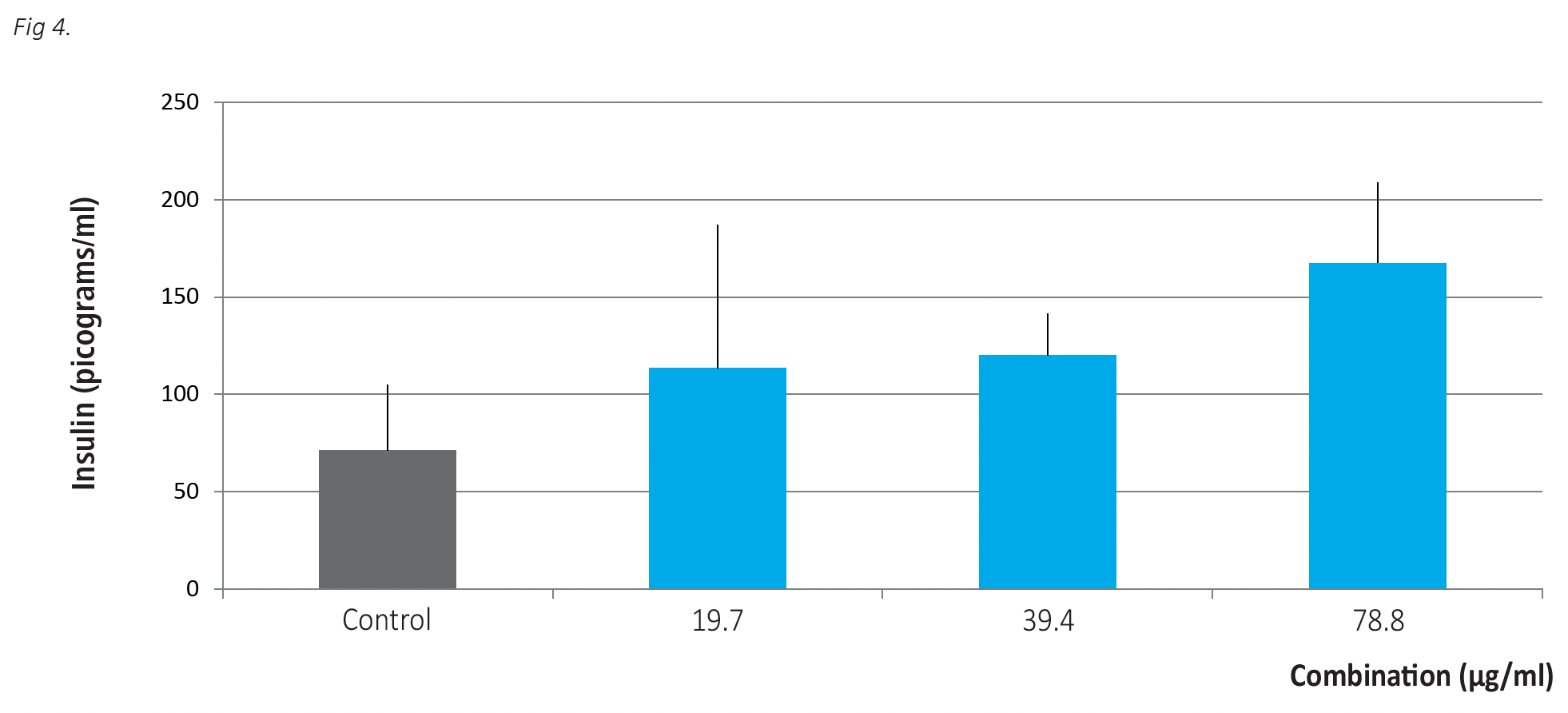
Fig 4. The effects of the combination of individual formulas on insulin secretion by human pancreatic cells after 24 hours incubation. Insulin was detected by ELISA as described in Materials and Methods. The results are expressed as average values +/-SD, obtained for three repetitions of each treatment.
REFERENCES
- https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html
- Cryer Wilson JD, Foster DW. Glucose homeostasis and hypoglycaemia. In William’s Textbook of Endocrinology., Eds. Philadelphia, Pa., W.B. Saunders Company, 1992, p.1223–1253
- Gerich JE, Langlois M, Noacco C, Karam JH, Forsham Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182(4108):171-173. doi:10.1126/science.182.4108.171
- Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt Diabetes. 2005; 54(8):2360-2364. doi:10.2337/diabetes.54.8.2360
- Chatterjee M, Ivanov V, Niedzwiecki A, Rath M: Micronutrient complexes support glucose metabolism in skeletal muscle J Cellular Medicine and Natural. Health 2019. May. (Available at: https://jcmnh.org/index.php/2019/05/13/micronutrient-complexes-support-glucose- metabolism-in-skeletal-muscle-cells)
- Rath Why Animals Don’t Get Heart Attacks … But People Do! Dr. Rath Health Foundation July 2018
- Miczke A, Suliburska J, Pupek-Musialik D, et al. Effect of L-arginine supplementation on insulin resistance and serum adiponectin concentration in rats with fat diet. Int J Clin Exp Med. 2015;8(7):10358-10366. 2015 Jul 15.
- González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, Ramírez-Ramírez V and Ramos-Zavala MG: Effect of thiamine administration on metabolic profile, cytokines and inflammatory markers in drug-naïve patients with type 2 Eur J Nutr. 2011; 50(2): 145-149. doi:10.1007/ s00394-010-0123-x
- Arora S, Lidor A, Abularrage CJ, et Thiamine (vitamin B1) improves endothelium-dependent vasodilatation in the presence of hyper glycemia. Ann Vasc Surg. 2006; 20(5): 653-658.
- Zygmunt K, Flaubert B, MacNeil J and Tsiani E. Naringenin, a citrus bioflavonoid, increases muscle cell glucose uptake via Biochem Biophys Res Commun. 2010; 398: 178-183. doi:10.1016/j.bbrc.2010.06.048
- Urios P, Grigorova-Borsos AM and Sternberg Flavonoids inhibit the formation of the cross-linking AGE pentosidine in collagen incubated with glucose, according to their structure. Eur J Nutr. 2007; 46: 139-146.
- Peng X, Cheng KW, Ma J, Chen B, Ho CT, Lo C, Chen F and Wang M: Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation J Agric Food Chem. 2008;56(6): 1907- 1911. doi:10.1021/jf073065v
- Suwannaphet W, Meeprom A, Yibchok-Anun S and Adisakwattana Preventive effect of grape seed extract against high-fructose diet-induced insulin resistance and oxidative stress in rats. Food Chem Toxicol. 2010; 48(7): 1853-1857. doi:10.1016/j.fct.2010.04.021
- Liu J, Sun H, Duan W, Mu D and Zhang L. Maslinic acid reduces blood glucose in KK-Ay Biol Pharm Bull. 2007; 30(11): 2075-2078. doi:10.1248/bpb.30.2075
- Yi X and Maeda N: Alpha-lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006; 55(8): 2238-2244. doi:10.2337/db06-0251
- Cha J, Ivanov V, Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M. Nutritional improvement of metabolic syndrome parameters in immature fructose-fed wild type mice. Mol Med Rep 2011;4(6):1053-1059. doi:10.3892/mmr.2011.562
- Sujatha P. Trace Elements in Diabetes Mellitus. Clin. Diagn. Res. 2013;7:1863–1865. doi:10.7860/JCDR/2013/5464.3335
- Fung, T., Manson, J. E., Solomon, C. G., Liu, S., Willett, W. C., & Hu, F. B. The association between magnesium intake and fasting insulin concentration in healthy middle-aged women. J Am Coll of Nutr. 2003; 22(6), 533–538. doi:10.1080/07315724.2003.10719332
- Barbagallo , Dominguez L.-J. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch. Biochem. Biophys. 2007;458(1):40–47. doi: 10.1016/j.abb.2006.05.007
- Yao X, Liu R, Li X, Li Y, Zhang Z, Huang S, Ge Y, Chen X, Yang X. Zinc, selenium and chromium co-supplementation improves insulin resistance by preventing hepatic endoplasmic reticulum stress in diet-induced gestational diabetes rats. Nutr. Biochem. 2021; 96: 108810. doi:10.1016/j.jnutbio.2021.108810
- Norouzi S, Adulcikas J, Sohal SS, Myers S , Zinc stimulates glucose oxidation and glycemic control by modulating the insulin signaling pathway in human and mouse skeletal muscle cell lines. PLoS ONE. 2018; 13(1): e0191727. https://doi.org/10.1371/journal.pone.0191727
- Yammine K, Wehbe R, Assi C. A systematic review on the efficacy of vitamin D supplementation on diabetic peripheral neuropathy. Clin Nutr. 2020; 39(10): 2970-2974. doi:10.1016/j.clnu.2020.01.022
- Ahmadieh H, Azar ST, Lakkis N, Arabi A. Hypovitaminsis d in patients with type 2 diabetes mellitus: a relation to disease control and ISRN Endocrinol. 2013;641098. doi:10.1155/2013/641098
- Fournet M, Bonté F, Desmoulière A: Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging Dis. 2018;9(5):880-900. Published 2018 Oct 1. doi: 10.14336/AD.2017.1121
- 25. Shu-Hsiang Huang, Sheng-Ting Fang and Yi-Cheng Chen. Molecular Mechanism of Vitamin K2 Protection against Amyloid-β-Induced Cytotoxicity. Biomolecules. 2021; 11(3), 423. Published 2021 Mar 13. https://doi.org/10.3390/biom11030423
- Mehta DS, Dound YA, Jadhav SS, Bhave AA, Devale M, Vaidya A novel potential role of Vitamin K2-7 in relieving peripheral neuropathy. J Pharmacol Pharmacother. 2018;9:180-5

