Peptide vaccines directed against human Metalloproteinases (MMPs) with anti-tumor efficacy in vitro and in vivo
Despite sporadic progress, cancer has thus far eluded preventive and therapeutic approaches that can lead to an effective control of this global epidemic. The single most important reason for this failure is the fact that no effective intervention became available to impede the pathological pathways common to all types of cancer. In our study, we targeted metalloproteinases (MMPS), endopeptidases that are involved in the breakdown of extracellular matrix (ECM). Among them, MMP-2 and MMP-9 are critically involved in all stages of cancer development including tumor growth, invasion and metastasis.
Our goal was to develop an anti-cancer vaccine by inhibiting these specific enzymes. We selected three oligopeptides from human MMP-9 and one from human MMP-2. We demonstrated the efficacy of these oligopeptides in generating immune response in mice. The in vitro tests showed that anti-MMPs sera were effective in curbing invasion of HeLA cells through the extracellular matrix system (Matrigel).
In our in vivo studies, we tested these oligopeptides for efficacy and safety in mice challenged with melanoma B16FO cells. All immunized animals injected with melanoma cancer cells grew smaller tumors than the control mice. The weight of tumors developed in the immunized animals was only about 30% of the controls, signifying a dramatic inhibition of tumor growth. The reduction in tumor volume varied between the peptide vaccines, but on average was even more pronounced. The highest reduction of tumor volume compared to control was 88% achieved with a vaccine derived from the human MMP-2 oligopeptide, followed by a reduction of 80% with one of the human MMP-9 oligopeptides. The weight gain of vaccinated and control animals was comparable throughout the study and no pathological side-effects were observed in the vaccinated animals.
If confirmed by further in vivo and later clinical studies, a vaccine for the prevention and treatment of all types of cancers – irrespective of their origin and type – may become available. Moreover, the limited economic burden of such a vaccine approach would allow its world-wide implementation towards the global control of cancer.
Introduction
At the beginning of the 21st century, cancer has remained a leading cause of death in many parts of the world, surpassed only by cardiovascular mortalities.[tooltip id=”tooltip_01″ title=”American Cancer Society, Global Cancer Facts & Figures, 3rd edition, 2015″]1[/tooltip] While recent developments of new therapeutic approaches to cancer offer hope for increased life expectancy for some forms of cancer, the results are moderate at best and the prohibitive costs of these therapies exclude them as an answer to the global cancer epidemic.
Despite these recent developments, chemotherapy and radiation have remained the standard cancer therapies in most countries. Thus, there is an urgent need for new effective, safe and affordable preventive and therapeutic approaches to the cancer epidemic.
Particularly disappointing in the search for such new approaches has been the lack of significant progress in addressing common pathways of cancerogenesis used by all types of cancers, irrespective of their origin and type.
Essentially all types of cancer use similar pathways for invasion and metastasis, which include the enzymatic destruction of connective tissue by matrix metalloproteinases (MMPs). In cancer pathogenesis, abnormally elevated levels of MMP enzymes enable tumor cells to digest the surrounding extracellular matrix, invade the adjacent connective tissue, and transmigrate through the endothelial layer of nearby blood capillaries to metastasize to other organs.[tooltip id=”tooltip_02″ title=”Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006; 25(1): 9-34. DOI: 10.1007/s10555-006-7886-9″]2[/tooltip]
MMPs are zinc dependent endopeptidases triggering the breakdown of extracellular matrix (ECM). This mechanism is not confined to cancer but is a basic biological mechanism involved in a multitude of physiological processes such as embryonic development, reproduction, tissue remodeling, and others. In healthy
conditions, this process remains under strict metabolic control.[tooltip id=”tooltip_03″ title=”Verma RP, Hansch C. Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q) SARs. Bioorg Med Chem. 2007; 15: 2223–2268. DOI: 10.1016/j.bmc.2007.01.011″]3[/tooltip] However, in cancer, chronic inflammation and other pathological conditions, the elevated stromal activity of MMPs − in particular MMP-2 and MMP-9 − escapes this metabolic regulation, thereby enabling uncontrolled extracellular matrix degeneration and facilitating progression of disease.[tooltip id=”tooltip_04″ title=”Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aneurysm progression. J Surg Res. 2007; 139(2): 292-307. DOI: 10.1016/j.jss.2006.09.020″]4[/tooltip],[tooltip id=”tooltip_05″ title=”Burrage PS, Mix KS. Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006; 11: 529-43. PMID: 16146751″]5[/tooltip],[tooltip id=”tooltip_06″ title=”Egeblad M, Werb Z. New functions for matrix metalloproteinases in cancer progression. Nature Rev Cancer. 2002; 2(3): 161-174. DOI: 10.1038/nrc745″]6[/tooltip],[tooltip id=”tooltip_07″ title=”Lindsey ML. MMP induction and inhibition in myocardial infarction. Heart Fail Rev. 2004; 9(1): 7-19. DOI: 10.1023/B:HREV.0000011390.44039.b7″]7[/tooltip],[tooltip id=”tooltip_08″ title=”Suzuki R, Miyazaki Y, Takagi K, Torri K, Taniguchi H. Matrix metalloproteinases in the pathogenesis of asthma and COPD: implications for therapy. Treat Resp Med. 2004; 3(1): 17-27. PMID: 15174890″]8[/tooltip]
In cancer, such uncontrolled activity of MMP-2 and MMP-9 correlates essentially with all stages of the neoplastic process, including tumor growth, invasion and metastasis. Moreover, MMPs promote vascular restructuring and tumor angiogenesis, a process essential to provide increased blood supply to the growing tumor.[tooltip id=”tooltip_09″ title=”Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gealtinase A-deficient mice. Cancer Res. 1998; 58(5): 1048-1051. PMID: 9500469 “]9[/tooltip],[tooltip id=”tooltip_10″ title=”Liu SC, Yang SF, Yeh KT et al. Relationship between the level of matrix metalloproteinase -2 and tumor size of breast cancer. Clin Chim Acta. 2006; 371(1-2): 92-96. DOI: 10.1016/j.cca.2006.02.026″]10[/tooltip],[tooltip id=”tooltip_11″ title=”Roomi MW, Monterey JC, Kalinovsky T, Niedzwiecki A, Rath M. Pattern of MMP2 and 9 expression in human cancer cell lines. Oncol Rep. 2009; 21(5): 1323-1333. PMID: 19360311″]11[/tooltip] In essence, MMP activity is a critical determinant of cancer malignancy and patient prognosis.[tooltip id=”tooltip_12″ title=”Fingleton B. Matrix metalloproteinase inhibitors for cancer therapy: the current situation and future prospects. Expert Opin Ther Tatgets. 2003; 7(3), 385-397. DOI: 10.1517/14728222.7.3.385″]12[/tooltip],[tooltip id=”tooltip_13″ title=”Vihinen P, Kähäri VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets, Int J Cancer. 2002; 99(2): 157-166. DOI: 10.1002/ijc.10329″]13[/tooltip]
The scientific quest to identify therapies that block the destructive effect of elevated MMP activity in the progression of cancer has been described as the ‘holy grail of medicine’. [tooltip id=”tooltip_14″ title=”Tansey B. Misdiagnosis / Failure of promising cancer treatment leads to soul searching by researchers, drug firms. San Francisco Chronicle. May 12, 2002, www.sfgate.com/business/article/Misdiagnosis-Failure-of-promising-cancer-2837479.php”]14[/tooltip] This characterization reflects the urgent pursuit of the international research community to identify a preventive and therapeutic target to control all types of cancer, irrespective of their origin, by blocking their common terminal pathway, metastasis.
Earlier attempts to develop synthetic pharmaceutical drugs against MMPs have failed due to severe side effects, and clinical trials with these drugs had to be terminated early.[tooltip id=”tooltip_12″ title=”Fingleton B. Matrix metalloproteinase inhibitors for cancer therapy: the current situation and future prospects. Expert Opin Ther Tatgets. 2003; 7(3), 385-397. DOI: 10.1517/14728222.7.3.385″]12[/tooltip],[tooltip id=”tooltip_15″ title=”Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002; 295(5564): 2387–92. DOI: 10.1126/science.1067100″]15[/tooltip],[tooltip id=”tooltip_16″ title=”Pavlaki M, Zucker S. Matrix Metalloproteinase Inhibitors (MMPIs): the beginning of Phase I or the termination of Phase III clinical trials. Cancer Metastasis Rev. 2003; 22(2-3): 177–203. PMID: 12784996″]16[/tooltip],[tooltip id=”tooltip_17″ title=”Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000; 19(56): 6642-50. DOI: 10.1038/sj.onc.1204097 “]17[/tooltip]
We are not aware of any reports in the scientific literature of a vaccine showing anti-cancer efficacy in vivo based on the inhibition of MMPs as decisive molecules in the spread of cancer.
In our study, we aimed at curbing, through vaccination, excessive activity of the MMP-2 and MMP-9 involved in cancer growth, invasion, metastasis, and angiogenesis. Here we report the results of testing three human MMP-9 oligopeptides and one human MMP-2 oligopeptide in vitro and in vivo. In vitro, we evaluated the ability of anti-MMPs sera in reducing cervical cancer HeLa cell invasion through a reconstructed basement membrane composed of extracellular matrix (Matrigel). The in vivo effect of vaccination with these MMP oligopeptides was tested by measuring their ability to limit tumor growth as determined by weight and volume of melanoma tumors in vaccinated mice versus controls.
Methods
Reagents
Complete Freund’s Adjuvant (Cat. No. F5881), Incomplete Freund’s Adjuvant (Cat. No. F5506), Avidin (Cat. No. A9275, Anti-mouse IgG-HRP conjugated antibody (Cat. No. A4416) and TMB substrate (Cat. No. T0440) were obtained from Sigma-Aldrich.
Murine melanoma B16F0 cells and HeLa cells obtained from ATCC® (CRL-6322™, American Type Culture Collection, Rockville, MD) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, 100 mcg/ml streptomycin. The media and serum were obtained from ATCC, while the antibiotics were from Gibco, BRL (Long Island, NY, USA).
Animals
All experimental procedures in the studies were approved by the Institutional Animal Ethics Committee (IAEC) of the Dr. Rath Research Institute. Male C57BL/6J mice of 5-6 weeks old were obtained from Simmonsens Laboratories (Gilroy, CA), USA. Animals were acclimatized for a period of two weeks prior to the study. Animals were maintained on a standard rodent diet ad libitum with free access to water under controlled conditions of temperature and humidity on a 12 h light-dark cycle.
Test peptides
The test MMP-9 and MMP-2 oligopeptides were synthesized at GenScript, NJ, USA. Each peptide of 90% purity was synthesized in two forms, one covalently conjugated to keyhole limpet hemocyanin (KLH) protein for immunization, the other conjugated to biotin for IgG ELISA. The peptides (6 mg) were dissolved in 6 mL of 0.1M NaHCO3 and aliquots stored at -20 ˚C until experimentation.
Sequence A #1
Human MMP-9: -C-H-F-P-F-I-F-EG-R-S-Y-S-A-C-
Sequence A #2
Human MMP-9: -D-T-D-D-R-F-G-F-
Sequence A#3
Human MMP-9: -D-R-D-K-L-F-G-F-C-P-T-R-A-D-S-
Sequence A#4
Human MMP-2: -C-P-R-K-P-K-W-D-K-C –
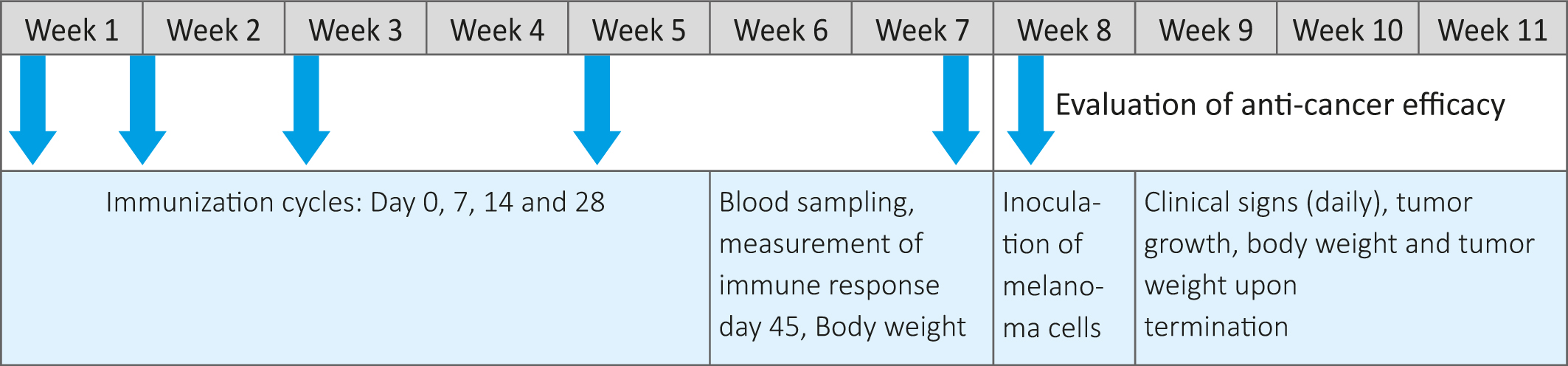
Preparation of peptide emulsion, immunization and evaluation schedule
On the day of immunization (Day0) 0.8 mL of peptide solution was gradually added to 0.8 mL of appropriate Freund’s adjuvant and simultaneously vortexed thoroughly to get a uniform emulsion. The animals in treatment groups ( -5 animals per group) were injected intraperitoneally with 100 ul solutions of the individual KLH conjugated peptides emulsified with Complete Freund ’s adjuvant (CFA). On Days 7, 14 and 28, animals were administered 100 µL of KLH conjugated peptides emulsified with Incomplete Freund’s Adjuvant (IFA). The control group were administered the adjuvant alone. The experimental timeline, including immunization, test and evaluation steps are presented in Table 1.
Group 1: Control (Untreated),
Group 2: Peptide sequence # A1
Group 3: Peptide sequence # A2
Group 4: Peptide sequence # A3
Group 5: Peptide sequence # A4
Blood sampling
On day 45, animals were anesthetized using Isoflurane and blood samples were withdrawn into 2ml microcentrifuge tubes and centrifuged at 5,000 rpm for 10 min at 4˚C for separation of serum. Samples were stored at -80˚C until analysis.
Immune response assay
The immune response was measured in serum samples using an antibody ELISA titer assay as described below. On day 1, 96-well microtiter plates (Maxisorp®, Nunc™) were coated with 100 µL of avidin solution (10 µg/mL prepared in 0.05 M carbonate buffer, pH 9.5) and incubated for 24 h at room temperature. On the next day, plates were washed 3 times with wash buffer (200 µL/well) followed by addition of 100 µL biotinylated peptide solution per well (50 µg/mL concentration prepared in Binding buffer) and incubated for 1h at 37˚C. Post incubation, the well contents were discarded and the plate was washed 3 times with wash buffer. The serum samples to be analyzed were serially diluted with binding buffer to get a concentration of 1:100, 1:1,000 and 1:10,000. 100 µL of the diluted serum samples were then added to the respective wells and incubated for 1h at 37˚C. After a serial wash with wash buffer, 100 µL of Anti-mouse IgG-HRP conjugated antibody diluted 1: 2000 in binding buffer was added to each well and incubated for 30 min at 37˚C. Plates were again washed 3 times with wash buffer, 100 µL of TMB substrate was added and incubated for 15 min at room temperature. The reaction was stopped by the addition of 100 µL of 0.3N HCl and the OD was measured at 450 nm in a microplate reader (Synergy™ HT, Biotek®). The IgG antibody titer was determined by quantification of color reaction and measured as OD units.
HeLa cells invasion assay
Invasion studies were conducted using Matrigel (Becton Dickinson) inserts in 24 well plates. Human HeLa cells (25,000/ insert in 0.2ml) were suspended in 0.2ml of 1:100 dilution of anti-sera of peptides A1, A2, A3 and A4 and seeded on to the insert in the well. Conditioned media from the fibroblasts was added to the wells. The plates with the inserts were then incubated in a culture incubator equilibrated with 95% air and 5% Co2 for 24 hrs. After incubation, the media from the wells were withdrawn. The cells on the upper surface of the insert were gently scrubbed away with cotton swabs. The cells that had penetrated the Matrigel membrane and migrated onto the lower surface of the Matrigel were stained with hematoxylin and eosin and counted under microscope, and percent inhibition was calculated compared to the control.
Evaluation of anti-tumor efficacy in mice model of B16FO melanoma
Murine melanoma B16FO cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% Fetal bovine serum. Sub-confluent cells monolayers were harvested, pelleted and re-suspended in growth medium prior to counting on a hemocytometer. Viable cells were counted using the Trypan blue exclusion method and a cell suspension with >90% viability was prepared in cold PBS. 0.2 mL of the cell suspension containing 0.5×106 cells were implanted subcutaneously in the immunized mice along with the control animals. Clinical signs were monitored daily and body weight was recorded twice weekly.
Tumor excision and gross pathology
After 3 weeks of cell implantation animals were sacrificed by CO2 asphyxiation. A necropsy was performed to examine gross pathology and metastasis. Tumors were excised and weight of the tumors was recorded and the pictures of the representative tumors were captured. Tumor volume was measured in terms of length (l) and width (w or b) by using a digital vernier caliper.
Table 1: Study design
Results
Antibody generation by test MMP oligopeptides
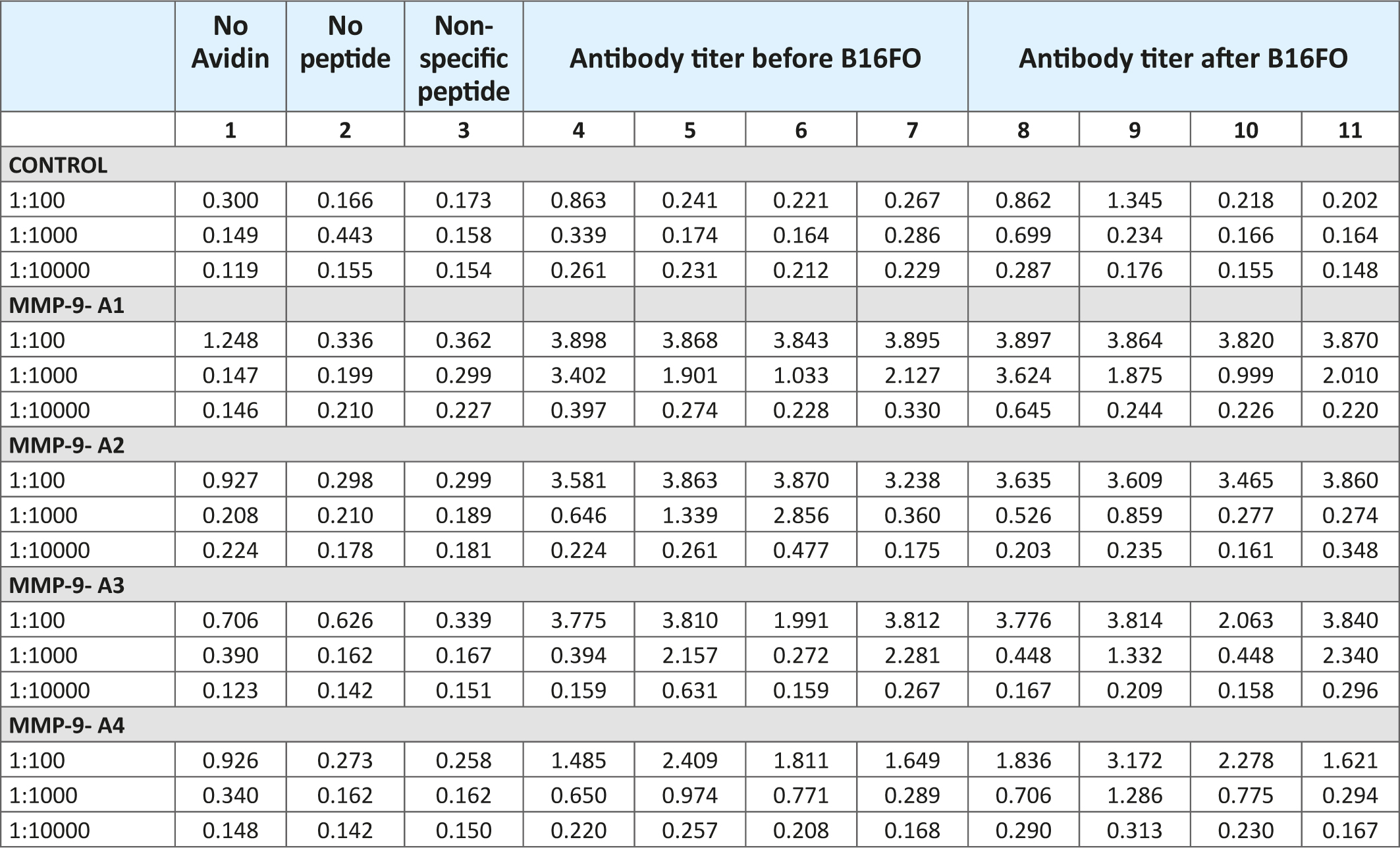
Table 2 presents the antibody response of mice immunized with the test oligopeptides A1 through A4 on day 45 post immunization and after the inoculation with B16FO melanoma cells. The results show that all immunized mice exhibited a significant elevation in serum IgG levels when compared to unimmunized animals and respective controls. The immune response was the highest at 1:100 serum dilutions and significantly decreased to almost control levels at 1:10,000. At 1:100 serum dilutions the immune response was higher for test MMP-9 oligopeptides (A1-through A3) compared to MMP-2 oligopeptide (A4). The results also indicate that after inoculation of mice with B16FO melanoma cells, the antibody titers did not change much compared to the pre-inoculation immune response.
Table 2: Immune response against human oligopeptides from MMP-9-A1, A2 and A3 and MMP-2-A4
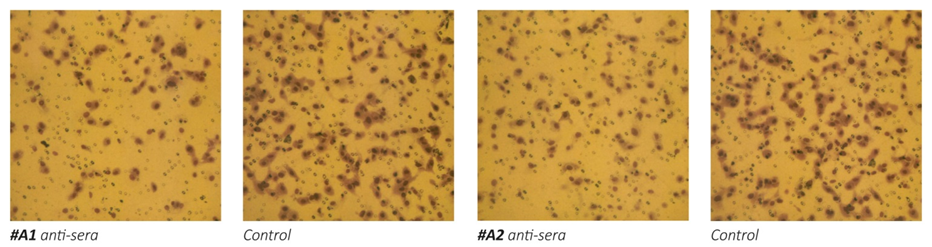
1a
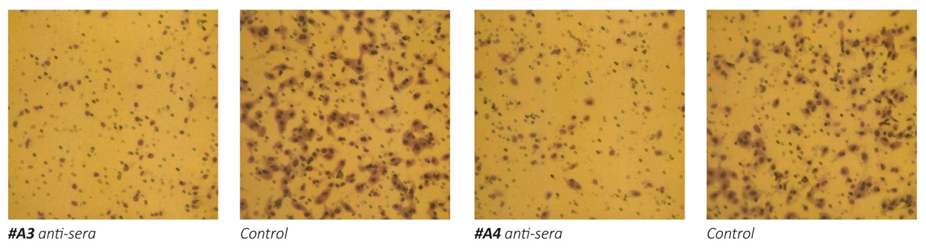
1b
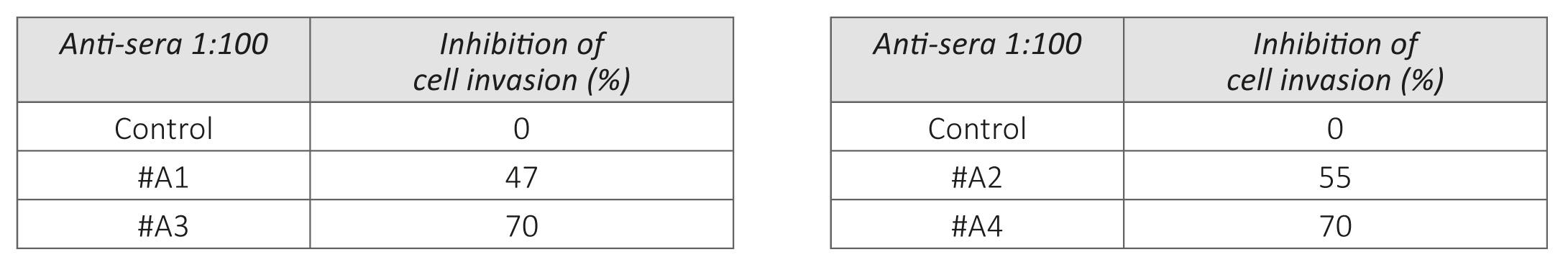
Figure 1 – Effect of anti-MMPs sera on Matrigel invasion of HeLa cells. 1a: micrographs of invading cells. 1b: Inhibition of cell invasion in %.
Anti-cancer efficacy of anti-MMP vaccines In Vitro: HeLa cell invasion
The results on Figure 1 a/, b- show that the exposure of human cervical cancer cells (HeLa) to the antisera obtained from animals immunized with oligopeptide sequences from human MMP-9 (A1, A2 and A3) and MMP-2 (A4) resulted in significant inhibition of these cells ability to invade Matrigel. The most effective in inhibiting HeLa cells extracellular matrix invasion were the antisera obtained from animals immunized with peptide A3 and peptide A4, which resulted in reducing cancer cells invasion by 70%. Sera obtained from mice immunized with two other MMP9 oligopeptides (A1 and A2) were also effective in inhibiting the invasiveness of cancer cells by 47% and 55%, respectively.
Anti-tumor effects of vaccination with human MMP-9 and MMP-2 oligopeptides: In Vivo evaluation
In addition, we evaluated the growth of melanoma B16FO tumors in mice immunized with MMP-9 and MMP-2 oligopeptides in vivo as determined by tumor weight and volume (Figure 2). Photographs of tumors developed in immunized mice are presented in Figure 3.
Mice immunized with oligopeptides from human MMP-9 and MMP-2 had a significantly reduced tumor weight by about 70% compared to control. The inhibition of a tumor weight was similar for all test oligopeptides (Figure 2A).
Tumor volume determination showed that mice immunized with MMP-2 oligopeptide grew tumors of the lowest tumor volume (88% reduction) compared to mice immunized with the three oligopeptides from MMP-9. Tumor volume in mice immunized with MMP 9 peptides A1, A2 and A3 was reduced by 70%, 80% and 68% respectively, compared to controls. Changes in tumor volume are presented in Figure 2B.
Remarkably, one out of four mice immunized with oligopeptide A2 and one out of the group immunized with oligopeptide A3 did not develop any tumor at all. It is a significant fact that the complete absence of tumor growth was observed in two separate groups of animals vaccinated with two distinct MMP-9 oligopeptides. In this pilot study this represents a complete suppression of tumor growth in 25% of the animals, an observation that deserves to be validated in larger settings.
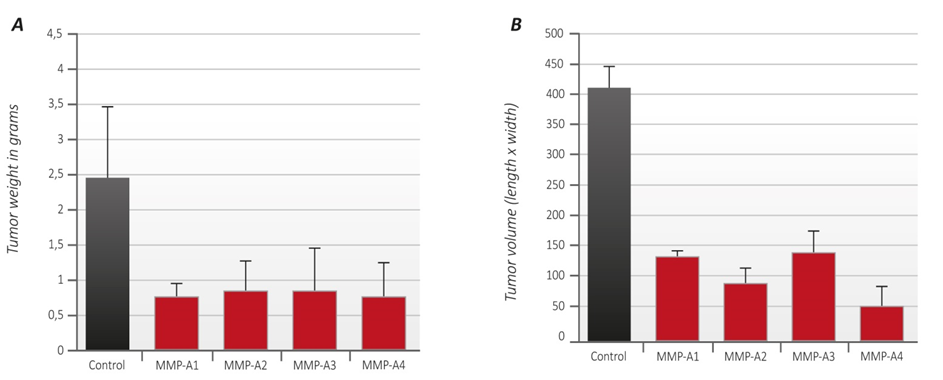
Figure 2 – A Tumor weight: The average tumor weight of unvaccinated animals (controls) is shown as black column. The average tumor weight from animals vaccinated with the oligopeptides A1, A2, A3 and A4 respectively, is represented by red columns.
– B Tumor volume: The average tumor volume of unvaccinated animals (controls) is represented by black column. The average tumor volume from animals vaccinated with the MMP oligopeptides A1, A2, A3 and A4 respectively, is represented by red columns.

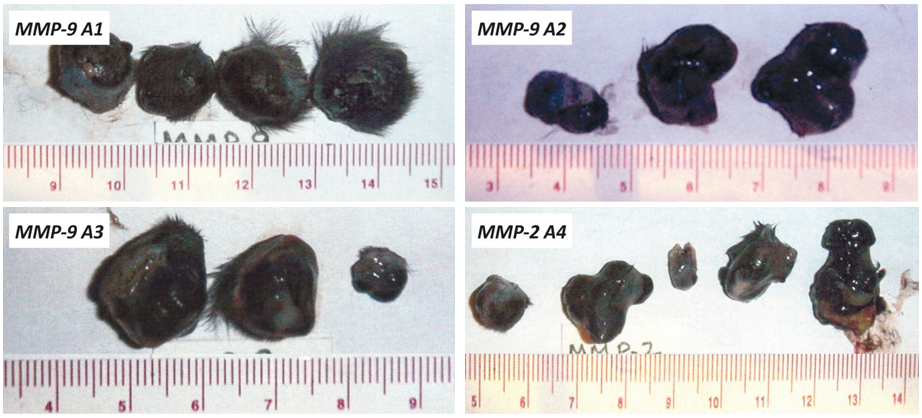
Figure 3 – Photographs of tumors developed in mice immunized with human MMP-9 and MMP-2 oligopeptides (A1-A4) and Control, A: Tumors from unvaccinated animals (control). B: Pictures of tumors from animals vaccinated with the MMP oligopeptides A1, A2, A3 and A4 respectively.
Evaluation of potential adverse effects
Body weight
The results on Figure 4 show that animals immunized with the MMP oligopeptides had a normal rate of weight gain, similar to the unvaccinated control animals. Moreover, there were no adverse health effects observed in the immunized mice during the course of the study. The macroscopic inspection of essential organs did not show any pathology.
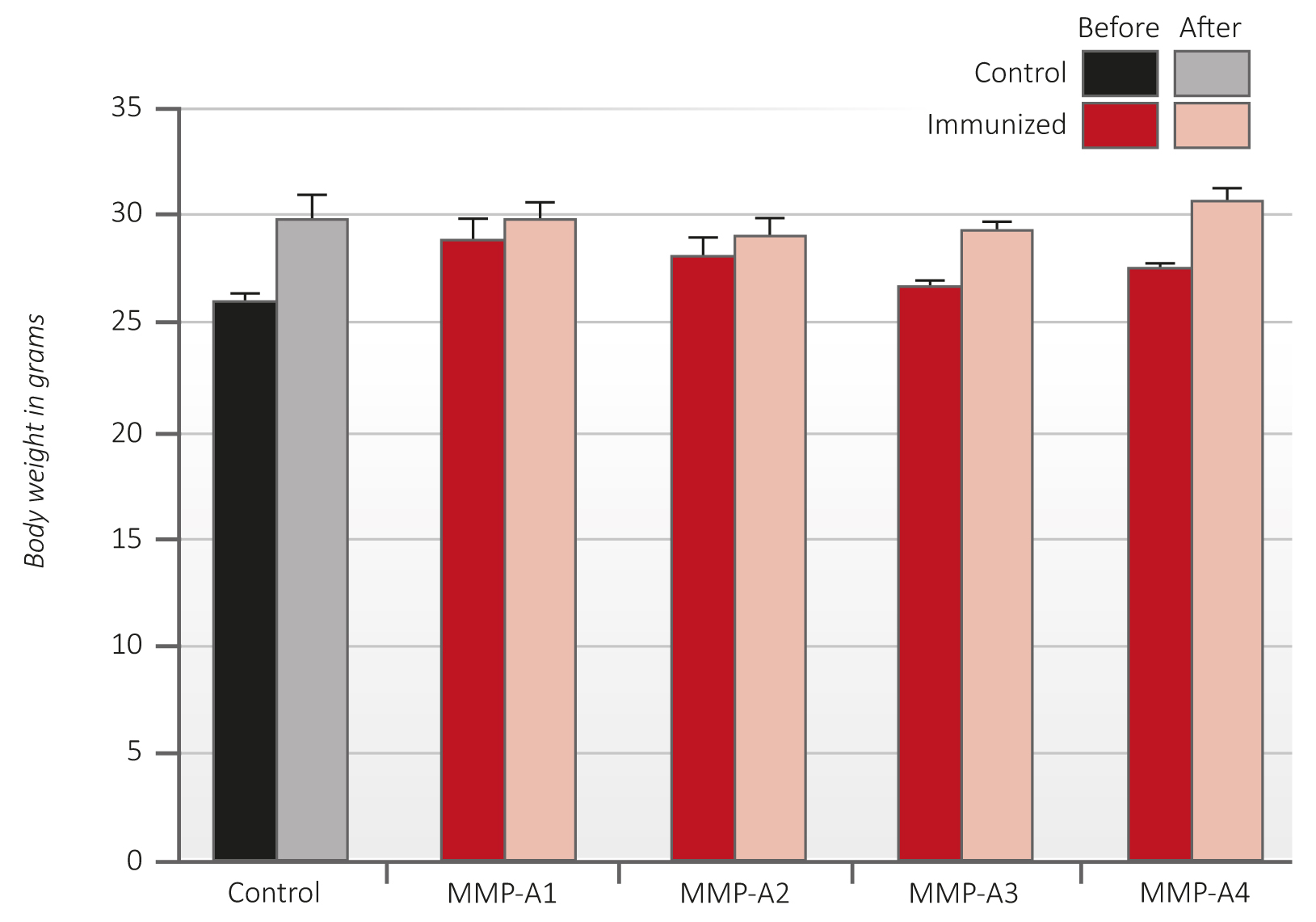
Figure 4 – Average body weight of C57Bl6/J mice immunized with peptides A1-A4 before (dark red columns) and after (light red columns) B16FO melanoma cells challenge in comparison to controls (black and grey).
Discussion
At the beginning of the 21st century, cancer has remained the second largest cause of death among humans, surpassed only by the mortality from cardiovascular disease. The reason for the continuation of this epidemic is that none of the therapeutic approaches developed thus far has met the necessary criteria for an effective control of this epidemic; namely the successful addressing of a defined biological target, safe and affordable manner.
Above all, any approach that promises to control cancer in the future must be able to successfully prevent the development of this disease by inhibiting the pathological pathways common to all cancer types, i.e. growth, invasive spread and metastasis.
None of the conventional approaches has thus far matched these criteria. Chemotherapy and radiation are unspecific therapeutic attempts and are frequently associated with severely harmful side effects. Newer therapeutic approaches based on immunological principles are more specific in addressing certain
types of cancer. [tooltip id=”tooltip_18″ title=”Cathcart J, Pulkoski-Gross A, Cao J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015; 2(1): 26-34. DOI: 10.1016/j.gendis.2014.12.002″]18[/tooltip],[tooltip id=”tooltip_19″ title=”Fields GB. New strategies for targeting matrix metalloproteinases. Matrix Biol. 2015; 44-46: 239-246. DOI: 10.1016/j.matbio.2015.01.002″]19[/tooltip] Certain monoclonal antibody therapies have shown some limited effects against specific cancers, but they are often unable to address a wider scope of cancer types and are economically challenging for both individual cancer patients and public health services alike [tooltip id=”tooltip_20″ title=”Schellekens H, Smolen JS, Dicato M, Rifkin RM. Safety and efficacy of biosimilars in oncology. Lancet Oncol. 2016;17(11): e502-e509 DOI: 10.1016/S1470-2045(16)30374-6″]20[/tooltip],[tooltip id=”tooltip_21″ title=”McKinnon R, Ward M. Safety considerations of biosimilars. Aust Prescr. 2016; 39(6): 188-189. DOI: 10.18773/austprescr.2016.084″]21[/tooltip],[tooltip id=”tooltip_22″ title=”Modjtahedi H, Ali S, Essapen S. Therapeutic application of monoclonal antibodies in cancer: advances and challenges. Br Med Bull. 2012;104: 41-59. DOI: 10.1093/bmb/lds032″]22[/tooltip],[tooltip id=”tooltip_23″ title=”Coulson A, Levy A, Gossell-Williams M. Monoclonal Antibodies in Cancer Therapy: Mechanisms, Successes and Limitations. West Indian Med J. 2014; 63(6): 650-4. DOI: 10.7727/wimj.2013.241″]23[/tooltip]
By the year 2020, it is estimated that the global market for such monoclonal antibodies against cancer will exceed US$40 billion. These exorbitant costs exclude the vast majority of global cancer patients from taking advantage of such therapies.
Besides these economic limitations, the even bigger flaw of such anti-cancer strategies, based on the infusion of monoclonal antibodies, is of a scientific nature. Monoclonal antibodies infused into the blood stream of a cancer patient do not take advantage of the power of the patient’s own immune system to create an immune response itself, like in vaccination.
Current antibody therapies offer a pre-formed immunological tool artificially created in the laboratories of drug companies. This approach requires that patients remain dependent on a continuous external supply of these patented antibodies in the form of expensive drug infusions – as opposed to letting the patient’s body continuously produce the antibodies itself, like with effective vaccinations.
The therapeutic approach shown in this publication takes the immunotherapy of cancer one step further by offering a potentially universal cancer vaccine. This approach does not use pre-manufactured antibodies but instead merely requires the injection of the antigen of a cancer-promoting molecule into the body. This then lets the body produce the cancer-fighting antibodies itself.
As therapeutic targets to test this approach in animals we picked MMP-2 and MMP-9, two enzymes that are known to catalyze decisive steps in cancer development that include tumor growth, invasion and metastasis.
Finding a therapeutic approach to neutralize the destructive effect of these MMPs on the connective tissue in cancer was already publicly described as the “holy grail of medicine” [tooltip id=”tooltip_14″ title=”Tansey B. Misdiagnosis / Failure of promising cancer treatment leads to soul searching by researchers, drug firms. San Francisco Chronicle. May 12, 2002, www.sfgate.com/business/article/Misdiagnosis-Failure-of-promising-cancer-2837479.php”]14[/tooltip] two decades ago. However, no therapeutic breakthrough in this direction has been accomplished thus far.
Here we report the effective use of human oligopeptides containing amino acid sequences of MMP-2 and MMP-9 – used as vaccines – in blocking cancer growth in mice challenged with murine melanoma cells without detectable adverse side effects (Figure 3). This effect was accompanied by a significant increase in the IgG antibody titers in the vaccinated animals compared to controls (Table 1).
The weight of tumors that developed in the immunized animals was about 70% lower compared to controls. Moreover, the reduction in volume of the tumors was on average even more pronounced than their weight. The highest inhibition of tumor volume was observed in mice immunized with MMP-2 oligopeptide (A4) by 88% and with MMP-9 oligopeptide (A2) by 80% respectively. Significantly, two mice in group A2 and A3 did not show any tumor growth at all, a fact that warrants particular evaluation in forthcoming larger experimental settings.
In our in vivo experiments, we tested the efficacy of human MMP-2 and MMP-9 oligopeptides in generating immune response and its anti-cancer effects against melanoma cancer cells (B16FO) originating from another species – mice. This cross-species efficacy is noteworthy, since the respective MMP oligopeptide sequences differ between humans and mice.
In our in vitro invasion essays – unlike in the in vivo experiments – we used cancer cells derived from a human cervix cancer cell line (HeLa). In these experiments, the efficacy of the sera from mice immunized with human MMP oligopeptides were tested for their ability to inhibit the invasion of cancer cells from the same species – humans. These anti-sera enriched in antibodies against human MMPs inhibited the ability of human cancer cells to digest connective tissue in the Matrigel test system, thereby curbing HeLa cancer cell invasion (Figure 1).
The inhibitory effect of the tested oligopeptides on human cancer cells in vitro – combined with their ability to curb cancer growth in in vivo tests – supports the potential value of these oligopeptides as promising candidates to fight human cancers and warrants an accelerated evaluation towards their potential clinical use.
As with all therapeutic approaches based on the interference of biological pathways, great care has to be taken that the molecules used as therapeutic agents – here, the antibodies induced by oligopeptide vaccines – do not negatively interfere with essential physiological processes. This is particularly important since certain metalloproteinases also play a role in healthy conditions. To that effect, we did a thorough macroscopic evaluation of the essential organs of the animals at autopsy and did not observe any abnormalities. Moreover, the weight development of all vaccinated animals was comparable to that of controls (Figure 4).
Continuous monitoring for potential side effects from the use of these oligopeptide vaccines remains an important aspect of the future development of this promising approach.
The novel therapeutic approach to cancer supported here in vitro and in vivo represents a promising lead towards a significant reduction of cancer. Particularly encouraging is the fact that the tested vaccines target pathological pathways common to all types of cancer and allow – for the first time – the perspective of a universal vaccine against all types of cancer.
Moreover, the costs for producing and offering these vaccines is a fraction of the costs of the current approaches to cancer including chemotherapy, radiation as well as antibody infusions, thus offering – for the first time – the perspective of an affordable approach towards the global control of cancer.
In our opinion, this perspective warrants an international scientific effort, supported by responsible governments and carried out by medical and scientific institutions committed to the goal of significantly reducing and eventually eliminating cancer as a threat to humanity.
We have patented [tooltip id=”tooltip_24″ title=”US patents # 8,003,110 and # 8,067,009, European Patent # EP 2 667 880 as well as other national and international patents.”]24[/tooltip] the technology described in this publication in many countries with the goal of allowing it to potentially become available – at affordable costs – to patients and nations around the world. We invite regional and national governments, public universities and other nonprofit organizations to join us in the further development of this promising tool to control cancer.
Acknowledgments
The authors gratefully acknowledge Ms. Nusrath Roomi for an excellent technical assistance. This study was supported by nonrestricted fund from Dr. Rath Health Foundation.
References
- American Cancer Society, Global Cancer Facts & Figures, 3rd edition, 2015. Summary of current scientific information about cancer.
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006; 25(1): 9-34. DOI: 10.1007/s10555-006-7886-9
- Verma RP, Hansch C. Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q) SARs. Bioorg Med Chem. 2007; 15: 2223–2268. DOI: 10.1016/j.bmc.2007.01.011
- Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aneurysm progression. J Surg Res. 2007; 139(2): 292-307. DOI: 10.1016/j.jss.2006.09.020
- Burrage PS, Mix KS. Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006; 11: 529-43. PMID: 16146751
- Egeblad M, Werb Z. New functions for matrix metalloproteinases in cancer progression. Nature Rev Cancer. 2002; 2(3): 161-174. DOI: 10.1038/nrc745
- Lindsey ML. MMP induction and inhibition in myocardial infarction. Heart Fail Rev. 2004; 9(1): 7-19. DOI: 10.1023/B:HREV.0000011390.44039.b7
- Suzuki R, Miyazaki Y, Takagi K, Torri K, Taniguchi H. Matrix metalloproteinases in the pathogenesis of asthma and COPD: implications for therapy. Treat Resp Med. 2004; 3(1): 17-27. PMID: 15174890
- Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gealtinase A-deficient mice. Cancer Res. 1998; 58(5): 1048-1051. PMID: 9500469
- Liu SC, Yang SF, Yeh KT et al. Relationship between the level of matrix metalloproteinase -2 and tumor size of breast cancer. Clin Chim Acta. 2006; 371(1-2): 92-96. DOI: 0.1016/j.cca.2006.02.026
- Roomi MW, Monterey JC, Kalinovsky T, Niedzwiecki A, Rath M. Pattern of MMP2 and 9 expression in human cancer cell lines. Oncol Rep. 2009; 21(5): 1323-1333. PMID: 19360311
- Fingleton B. Matrix metalloproteinase inhibitors for cancer therapy: the current situation and future prospects. Expert Opin Ther Tatgets. 2003; 7(3), 385-397. DOI: 10.1517/14728222.7.3.385
- Vihinen P, Kähäri VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets, Int J Cancer. 2002; 99(2): 157-166. DOI: 10.1002/ijc.10329
- Tansey B. Misdiagnosis / Failure of promising cancer treatment leads to soul searching by researchers, drug firms. San Francisco Chronicle. May 12, 2002, www.sfgate.com/business/article/Misdiagnosis-Failure-of-promising-cancer-2837479
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. 2002; 295(5564): 2387–92. DOI: 10.1126/science.1067100
- Pavlaki M, Zucker S. Matrix Metalloproteinase Inhibitors (MMPIs): the beginning of Phase I or the termination of Phase III clinical trials. Cancer Metastasis Rev. 2003; 22(2-3): 177–203. PMID: 12784996
- Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. 2000; 19(56): 6642-50. DOI: 10.1038/sj.onc.1204097
- Cathcart J, Pulkoski-Gross A, Cao J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015; 2(1): 26-34. DOI: 10.1016/j.gendis.2014.12.002
- Fields GB. New strategies for targeting matrix metalloproteinases. Matrix Biol. 2015; 44-46: 239-246. DOI: 10.1016/j.matbio.2015.01.002
- Schellekens H, Smolen JS, Dicato M, Rifkin RM. Safety and efficacy of biosimilars in oncology. Lancet Oncol. 2016;17(11): e502-e509 DOI: 10.1016/S1470-2045(16)30374-6
- McKinnon R, Ward M. Safety considerations of biosimilars. Aust Prescr. 2016; 39(6): 188-189. DOI: 10.18773/austprescr.2016.084
- Modjtahedi H, Ali S, Essapen S. Therapeutic application of monoclonal antibodies in cancer: advances and challenges. Br Med Bull. 2012;104: 41-59. DOI: 10.1093/bmb/lds032
- Coulson A, Levy A, Gossell-Williams M. Monoclonal Antibodies in Cancer Therapy: Mechanisms, Successes and Limitations. West Indian Med J. 2014; 63(6): 650-4. DOI: 10.7727/wimj.2013.241
- US patents # 8,003,110 and # 8,067,009, European Patent # EP 2 667 880 as well as other national and international patents.
Supplemental materials
Rationale of anti-cancer vaccine development based on MMP peptides
Table 1: Study design

Table 2: Immune response against human oligopeptides from MMP-9-A1, A2 and A3 and MMP-2-A4




Figure 1 – Effect of anti-MMPs sera on Matrigel invasion of HeLa cells. 1a: micrographs of invading cells. 1b: Inhibition of cell invasion in %.

Figure 2 – A Tumor weight: The average tumor weight of unvaccinated animals (controls) is shown as black column. The average tumor weight from animals vaccinated with the oligopeptides A1, A2, A3 and A4 respectively, is represented by red columns.
– B Tumor volume: The average tumor volume of unvaccinated animals (controls) is represented by black column. The average tumor volume from animals vaccinated with the MMP oligopeptides A1, A2, A3 and A4 respectively, is represented by red columns.


Figure 3 – Photographs of tumors developed in mice immunized with human MMP-9 and MMP-2 oligopeptides (A1-A4) and Control, A: Tumors from unvaccinated animals (control). B: Pictures of tumors from animals vaccinated with the MMP oligopeptides A1, A2, A3 and A4 respectively.

Figure 4 – Average body weight of C57Bl6/J mice immunized with peptides A1-A4 before (dark red columns) and after (light red columns) B16FO melanoma cells challenge in comparison to controls (black and grey).
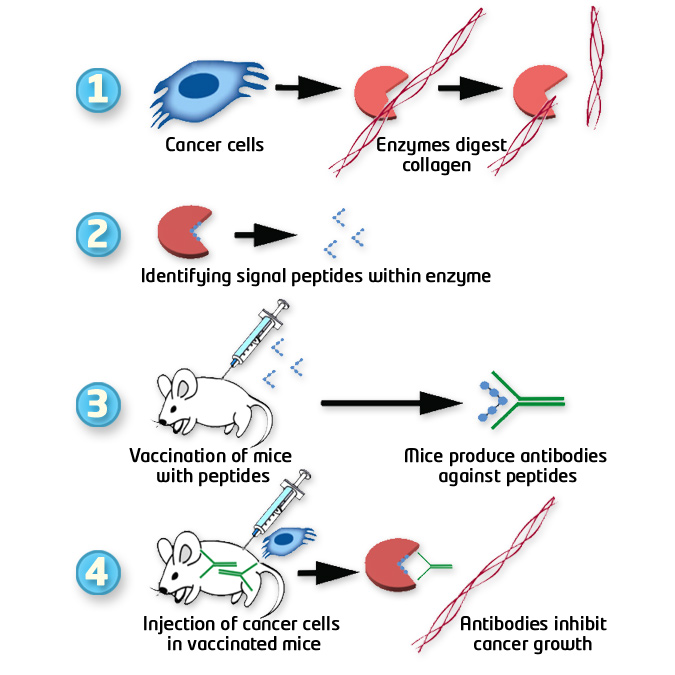
Rationale of anti-cancer vaccine development based on MMP peptides
References
- American Cancer Society, Global Cancer Facts & Figures, 3rd edition, 2015. Summary of current scientific information about cancer.
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006; 25(1): 9-34. DOI: 10.1007/s10555-006-7886-9
- Verma RP, Hansch C. Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q) SARs. Bioorg Med Chem. 2007; 15: 2223–2268. DOI: 10.1016/j.bmc.2007.01.011
- Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aneurysm progression. J Surg Res. 2007; 139(2): 292-307. DOI: 10.1016/j.jss.2006.09.020
- Burrage PS, Mix KS. Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006; 11: 529-43. PMID: 16146751
- Egeblad M, Werb Z. New functions for matrix metalloproteinases in cancer progression. Nature Rev Cancer. 2002; 2(3): 161-174. DOI: 10.1038/nrc745
- Lindsey ML. MMP induction and inhibition in myocardial infarction. Heart Fail Rev. 2004; 9(1): 7-19. DOI: 10.1023/B:HREV.0000011390.44039.b7
- Suzuki R, Miyazaki Y, Takagi K, Torri K, Taniguchi H. Matrix metalloproteinases in the pathogenesis of asthma and COPD: implications for therapy. Treat Resp Med. 2004; 3(1): 17-27. PMID: 15174890
- Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gealtinase A-deficient mice. Cancer Res. 1998; 58(5): 1048-1051. PMID: 9500469
- Liu SC, Yang SF, Yeh KT et al. Relationship between the level of matrix metalloproteinase -2 and tumor size of breast cancer. Clin Chim Acta. 2006; 371(1-2): 92-96. DOI: 0.1016/j.cca.2006.02.026
- Roomi MW, Monterey JC, Kalinovsky T, Niedzwiecki A, Rath M. Pattern of MMP2 and 9 expression in human cancer cell lines. Oncol Rep. 2009; 21(5): 1323-1333. PMID: 19360311
- Fingleton B. Matrix metalloproteinase inhibitors for cancer therapy: the current situation and future prospects. Expert Opin Ther Tatgets. 2003; 7(3), 385-397. DOI: 10.1517/14728222.7.3.385
- Vihinen P, Kähäri VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets, Int J Cancer. 2002; 99(2): 157-166. DOI: 10.1002/ijc.10329
- Tansey B. Misdiagnosis / Failure of promising cancer treatment leads to soul searching by researchers, drug firms. San Francisco Chronicle. May 12, 2002, www.sfgate.com/business/article/Misdiagnosis-Failure-of-promising-cancer-2837479
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. 2002; 295(5564): 2387–92. DOI: 10.1126/science.1067100
- Pavlaki M, Zucker S. Matrix Metalloproteinase Inhibitors (MMPIs): the beginning of Phase I or the termination of Phase III clinical trials. Cancer Metastasis Rev. 2003; 22(2-3): 177–203. PMID: 12784996
- Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. 2000; 19(56): 6642-50. DOI: 10.1038/sj.onc.1204097
- Cathcart J, Pulkoski-Gross A, Cao J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015; 2(1): 26-34. DOI: 10.1016/j.gendis.2014.12.002
- Fields GB. New strategies for targeting matrix metalloproteinases. Matrix Biol. 2015; 44-46: 239-246. DOI: 10.1016/j.matbio.2015.01.002
- Schellekens H, Smolen JS, Dicato M, Rifkin RM. Safety and efficacy of biosimilars in oncology. Lancet Oncol. 2016;17(11): e502-e509 DOI: 10.1016/S1470-2045(16)30374-6
- McKinnon R, Ward M. Safety considerations of biosimilars. Aust Prescr. 2016; 39(6): 188-189. DOI: 10.18773/austprescr.2016.084
- Modjtahedi H, Ali S, Essapen S. Therapeutic application of monoclonal antibodies in cancer: advances and challenges. Br Med Bull. 2012;104: 41-59. DOI: 10.1093/bmb/lds032
- Coulson A, Levy A, Gossell-Williams M. Monoclonal Antibodies in Cancer Therapy: Mechanisms, Successes and Limitations. West Indian Med J. 2014; 63(6): 650-4. DOI: 10.7727/wimj.2013.241
- US patents # 8,003,110 and # 8,067,009, European Patent # EP 2 667 880 as well as other national and international patents.

