Micronutrient combination inhibits two key steps of coronavirus (SARS-CoV-2) infection: viral binding to ACE2 receptor and its cellular expression
Introduction:The severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) infection that causes coronavirus disease 2019 (COVID-19), declared by WHO as a pandemic in 2020, poses a challenge to human health as well as to global economies. Thus far, the quest for vaccines and other approaches to this pandemic have focused on synthetic molecules, which are – predictably – associated with a variable degree of adverse side effects.
Results: Here we report that a combination of specific micronutrients can block the interaction between the binding site of SARS-CoV-2 and its cellular anchor, the ACE2 receptor. Moreover, this micronutrient combination was able to significantly decrease the expression of the ACE2 receptor on human alveolar (lung) epithelial cells by more than 90%.r 90%.
Conclusion: This study provides the basis for an effective and safe public health strategy based on optimum intake of micronutrients. It allows people around the world to actively participate in the prevention of coronavirus infections – beyond wearing masks, social distancing and other defensive measures.
Introduction
The rapid spread of the current coronavirus pandemic (COVID-19) is threatening global health, debilitating the economies of the world, and challenging the prosperity of future generations.1 In the first half of 2020, COVID-19 affected more than 16 million people and caused more than 660,000 deaths worldwide, as declared by WHO.2
By sequencing the whole genome of a virus from patient samples, Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) was identified, and the disease caused by this virus was named coronavirus disease 2019 (COVID-19).3
A major step towards curbing the spread of this pandemic was the identification of the pathway by which SARS-CoV-2 infects the cells of the human body. As reported by Zhou et al., Ou et al., and Hoffman et al., SARS-CoV-2 first binds to specific receptor on the surface of cell.4,5,6 Subsequently, the virus enters the cell and is carried via transport vesicles (endosomes) to the cell core (nucleus), where its genetic material is incorporated into the DNA.7
Of particular importance is the first step on this infective pathway, namely the binding of the virus to the receptor on the cell surface (Figure 1). This binding mechanism involves a specific spike protein, anchored on the surface of the SARS-CoV-2 and containing a receptor-binding domain (RBD) that specifically recognizes its ‘docking place’ – a specific receptor on the surface of the body cells. This receptor, called angiotensin-converting enzyme 2 (ACE2), is an integral membrane protein present on many cells throughout the human body, with its strong expression in the heart, vascular system, gastrointestinal system and kidneys, as well as in type II alveolar cells in the lungs.8
Since, this binding mechanism is an important determinant of viral infectivity, it is a major target in the development of vaccines and therapeutics.9,10
While the search for such interventional drugs and vaccines is ongoing, there could yet be another way to prevent this interaction of SARS-CoV-2 with the ACE2 receptor: by suppression of the expression of the ACE2 receptors on human body cells, so they are no longer available for viral anchoring.
In our recent pioneering study, we were able to show that a specific combination of micronutrients containing vitamin C, certain minerals, amino acids, and plant extracts, was effective in significantly decreasing cellular ACE2 expression in key types of cells targeted by the SARS- CoV-2: human lung small airways (alveolar) epithelial cells and human blood vessel (vascular) endothelial cells. These micronutrients were particularly effective in down-regulating the ACE2 receptor expression under inflammatory conditions, which are associated with SARS- CoVs infections.11
The remarkable results of this study triggered the question as to whether micronutrients are also able to affect another mechanism essential for SARS-CoV-2 infection, namely the viral binding to its cognate ACE2 receptor, the very same pathway targeted by essentially all vaccine- based approaches.
If the results of this study were positive, a new, natural and safe approach would become available to humankind to effectively control the current pandemic.
Thus, in the present study, we tested the efficacy of another nutrient combination, containing polyphenols and plant components, on both key aspects of SARS- CoV-2 infectivity: inhibiting the expression of cellular ACE2 receptor and – at the same time – blocking of the binding of a SARS-CoV-2 spike protein to the ACE2 receptor on human cells.
Methods
Cell cultures
Human small airways epithelial cells (SAEC) were cultured in airways epithelial cells growth medium (ATCC) in plastic flasks at 37oC and 5% CO2. In the experiments SAEC, passage 5-7, were plated to collagen-covered 96-well plates in 100 μl growth medium and were grown for 4-7 days to reach confluent layer.
Micronutrient composition
The micronutrient combination used in our experiments was developed at the Dr. Rath Research Institute (San Jose, CA). The test formulation contained: quercetin – 400 mg, cruciferous plant extract – 400 mg, turmeric root extract – 300 mg, green tea extract (80% polyphenols) – 300 mg, and resveratrol – 50 mg.
Cell supplementation
The micronutrient mixture was dissolved in 0.1N HCl, according to US Pharmacopeia protocol (USP 2040), and designated as a stock solution. For ACE2 expression experiments, SAEC cells were supplemented with indicated doses of the formulation in cell growth medium at 100 μl/well for 3-7 day. Applied nutrient concentrations were expressed in μg/ml.
ACE-2 ELISA assay
Culture plate wells were washed twice with phosphate- buffered saline (PBS) and fixed with 3% formaldehyde/0.5% Triton X-100/PBS solution for 1h at 4oC, then washed four times with PBS. Subsequently, 200 μl of 1% bovine serum albumin (BSA, Sigma) in PBS was added and the plates were incubated at 4oC overnight. Rabbit polyclonal anti ACE-2 antibodies (Sigma) were added to 100 μl 1% BSA/PBS for 1.5 h incubation at room temperature (RT). After three wash cycles with 0.1% BSA/PBS the wells were supplied with 100 μl anti-rabbit IgG antibodies conjugated with horseradish peroxidase (HRP, Sigma) for 1h at RT. After three wash cycles with 0.1% BSA/PBS the retained HRP activity was determined by incubation with 100 μl TMB substrate solution (Sigma) for 20 min at RT, followed by the addition of 50 μl of 1N H2SO4, and optical density measurements were conducted at 450 nm with microplate reader (Molecular Devices). Results are expressed as a percentage of experimental addition- free control (mean +/- SD, n=6). Non-specific control (wells incubated without anti-ACE2 antibodies) mean value (n=6) was subtracted from all sample values.
RBD (receptor-binding domain) blocking
This assay was performed using GenScript (Piscataway, NJ) SARS-CoV-2 surrogate virus neutralization test kit, which can detect both antibodies and inhibitors that block the interaction between the receptor-binding domain (RBD) of the viral spike protein of SARS-CoV-2 with human ACE2 cell surface receptor.
All test samples, with indicated concentrations, and positive and negative controls (provided by the manufacturer), were diluted with the sample dilution buffer with a volume ratio of 1:9. In separate tubes, HRP-conjugated RBD was also diluted with the HRP dilution buffer with a volume ratio of 1:99.
Binding/neutralization reaction was performed according to the manufacturer’s protocol. Briefly, diluted positive and negative controls as well as the test samples with indicated concentrations were mixed with the diluted HRP- RBD solution with a volume ratio of 1:1 and incubated for 30 min in 37oC. Next, 100 µl each of the positive control mixture, negative control mixture, and the test sample mixtures were added to the corresponding wells with immobilized ACE2 receptor and incubated for 15 min at 37oC. Subsequently, the plates were washed four times with 260 µl/well of the 1 x wash solution, and TMB solution was added to each well (100 µl/well). Plates were incubated in the dark at room temperature for up to 5 min. Next, 50 µl/ well of stop solution was added to quench the reaction, and the absorbance was measured immediately in plate reader at 450 nm. The experiment was performed three times in duplicates. Data are presented as % of control.
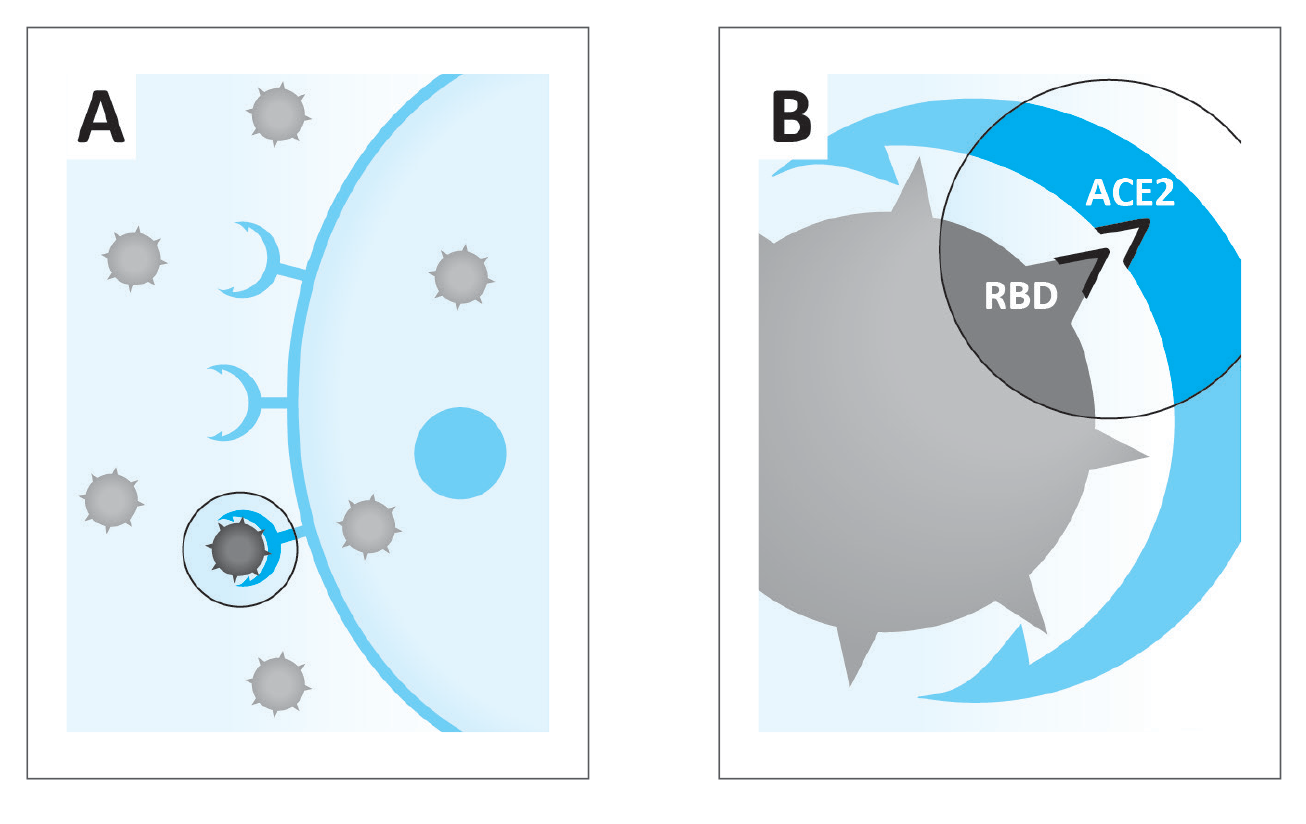
Figure 1: A. Coronaviruses infect human body cell via the ACE2 receptor.
B. Principle of the commercially available research kit used in this study to test the efficacy of micronutrients combination in blocking attachment of the receptor binding domain (RBD) of the SARS-CoV-2 to the ACE2 receptor on the cell surface.
Results
Efficacy of a specific micronutrient combination on ACE2 expression in human small alveolar epithelial cells.
Figure 2 shows the effect of different concentrations of a specific combination of various active plant components and extracts on cellular expression of ACE2 receptors in human small alveolar epithelial cells.
The results reveal a concentration-dependent decrease in cellular ACE2 receptor expression evaluated by a specific antibody binding. At the highest nutrient concentration of 320 µg/ml, the expression of cellular ACE2 receptors decreased by 92%. This indicates that, in the presence of these micronutrients, the viral binding to the cell surface can be substantially reduced.

Figure 2: Effects of micronutrient combination on ACE2 receptor expression in human small alveolar epithelial cells. Changes in ACE2 expression are presented as % of control.
The effect of specific micronutrient combination on SARS-CoV-2 RBD binding to ACE2 receptor
Binding of the RBD sequence of the spike protein of the SARS-CoV-2 to its cellular receptor is the necessary step in gaining access by this virus to the target cells and for viral infectivity.
In our study we applied a state-of-the-art, high- sensitivity-screening test that can identify various inhibitors that block the interaction between the receptor-binding domain (RBD) of the viral spike protein with human ACE2 receptor.
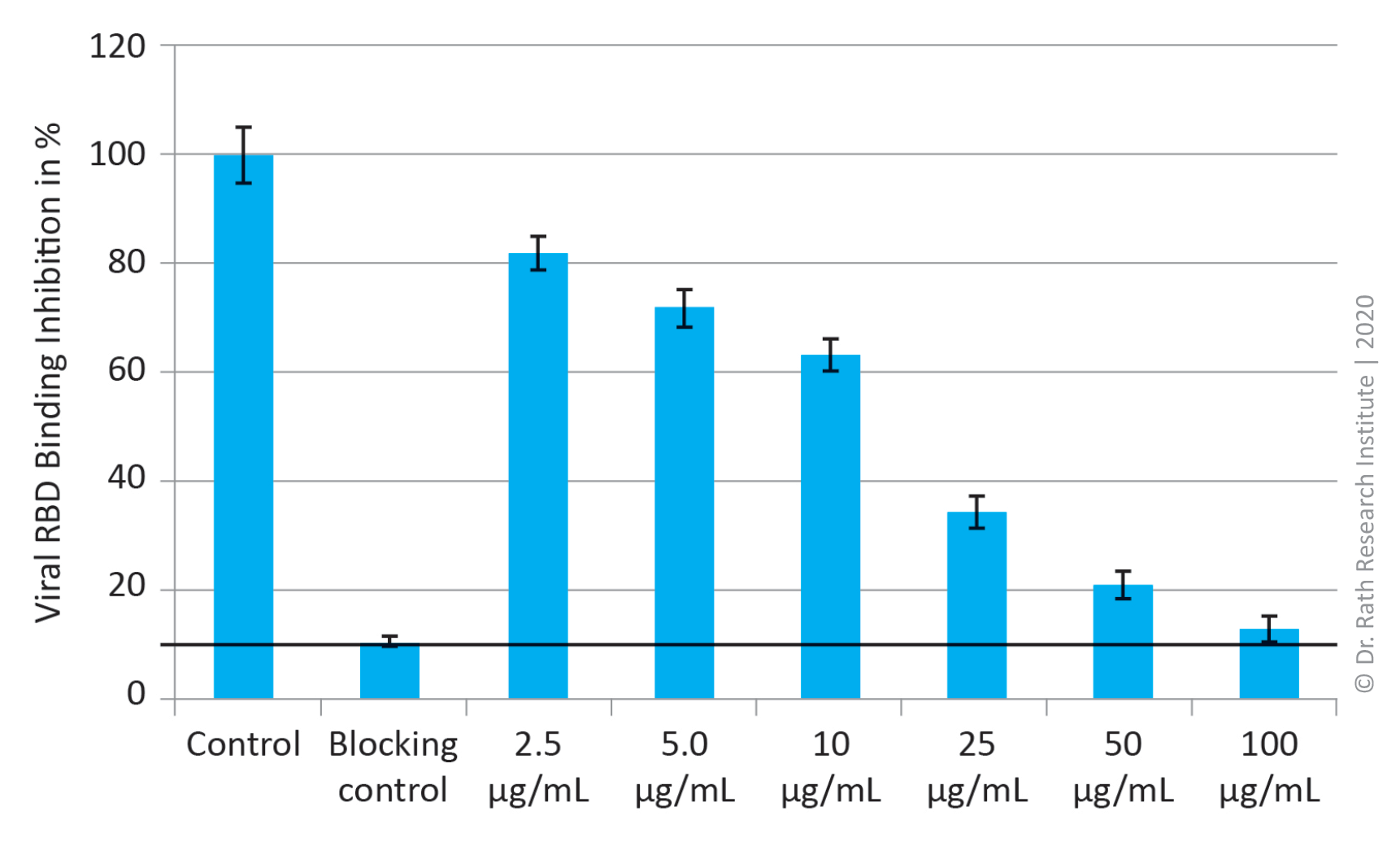
Figure 3: Blocking of SARS-CoV-2 spike protein (RDB) binding to human ACE2 receptor by specific micronutrient combination. Results are presented as % of control. ‘Blocking control’ = 100% binding inhibition.
Figure 3 shows that the same micronutrients combination of natural plant-derived compounds was able to block the attachment of the RBD of the spike protein of the SARS-CoV-2 virus to its cognate ACE2 receptor. This inhibitory effect was concentration dependent and at 100 µg/ml this mixture caused 97% binding inhibition. Its strong efficacy in preventing viral binding was observed even at a 40-times-lower concentration, i.e., 2.5 µg/ml, which caused about 20% binding inhibition.
Discussion
Significance of our findings
The results presented in this study document that micronutrients could strongly inhibit important cellular mechanisms associated with SARS-CoV-2 infection.
These data become available at a critical time when the global scientific and medical community is desperately searching for effective solutions to the COVID-19 pandemic.
By applying scientific methodologies and state-of- the-art techniques used in coronavirus research, we have shown that micronutrients can inhibit the binding of the RBD of the spike protein of SARS-CoV-2 to its specific receptor ACE2 by 97%. This means that micronutrients could almost completely prevent
viral infectivity. In addition, the same micronutrient combination inhibited the expression of ACE2 receptors on human small alveolar epithelial cells by up to 92%.
The present study indicates that specific formulations of plant-derived, biologically active compounds can be effective in simultaneous controlling critical mechanisms involved in the infectivity of the SARS- CoV-2.
In addition, as mentioned above, we also reported that another combination of natural compounds, including vitamin C, amino acids, plant components and minerals, could significantly decrease the expression of ACE2 receptors on two types of cells mainly targeted by the SARS-CoVs: human lung epithelial and human lung vascular endothelial cells.11 Another important finding of that study was that inhibition of ACE2 receptors expression was even more pronounced under pro-inflammatory conditions that accompany any viral infection. This would imply that the efficacy of micronutrients would be even more noticeable in clinical conditions and advanced stages of COVID-19, characterized by generalized inflammation and a so- called ‘cytokine storm’.
In the light of our findings, it is particularly disappointing that scientific efforts have thus far largely ignored the large body of scientific and clinical evidence that substantiates the efficacy of micronutrients in many aspects of viral infections. 12,13,14,15
The evidence that natural compounds such as micronutrients could be the answer to SARS-CoV-2 and other viral infections should inspire the scientific and medical community to embark on a global effort to expand the knowledge about the therapeutic value of micronutrients and other natural compounds in the prevention of infectious diseases in general.
Implications of these findings for public health policies
The magnitude of the current pandemic and the dimension of its human and economic costs makes our documentation of the specific value of micronutrients in controlling this pandemic a compelling public health approach.
This is particularly important since all other measures currently developed are either associated with severe side effects or are novel and untested strategies. The cautionary reactions of the international community to the “next generation” vaccines, which are DNA/RNA- based, testify to the awareness of the potential risks associated with a population-wide implementation of such technologies.
Based on our study results, a scientifically proven micronutrient-based approach becomes available as an effective – and safe – public health strategy to fight the current pandemic. With a blocking rate of nearly 100%, micronutrients rival the efficacy of any vaccine – yet without any incalculable risks.
A micronutrient-based approach also allows the people at large to actively participate in the prevention of infections caused by coronaviruses – beyond wearing masks, social distancing and other defensive measures.
References
- Chakraborty I, Maity COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci Total Environ. 2020; 728: 138882. https://dx.doi.org/10.1016%2Fj.scitotenv.2020.138882
- WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/. Updated August 13, Accessed August 14, 2020.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020; 382(8): 727-733. doi: 1056/NEJMoa2001017
- Zhou P, Yang XG, Hu B, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579(7798): 270–273. doi: 10.1038/s41586-020-2012-7
- Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature Commun. 2020; 11(1): 1620. doi: 10.1038/ s41467-020-15562-9
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020; 181(2):271-280.e8 doi: 10.1016/j.cell.2020.02.052
- Li Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016; 3(1): 237-261. doi:10.1146/annurev-virology-110615-042301
- Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl Acad. Sci. USA. 2005; 102(22):7988– 7993. https://doi.org/10.1073/pnas.0409465102
- Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The Spike protein of SARS-CoV—A target for vaccine and therapeutic. Nat. Rev. Microbiol. 2009; 7, 226–236. https://doi.org/10.1038/nrmicro2090
- Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang. MERS-CoV Spike protein: a key target for antivirals. Expert Opin Ther Targets. 2017; 21(2): 131-143. doi:10.1080/14728222.2017.1271415
- Ivanov V, Ivanova S, Niedzwiecki A, Rath M. Effective and save global public health strategy to fight the COVID-19 pandemic: Specific micronutrient combination inhibits Coronavirus cell-entry receptor (ACE2) expression. J Cell Med & Nat. Health, 2020.
- Jariwalla RJ, Roomi MW, Gangapurkar B, Kalinovsky T, Niedzwiecki A, Rath M. Suppression of influenza A virus nuclear antigen production and neuraminidase activity by a nutrient mixture containing ascorbic acid, green tea extract and amino acids. 2007; 31(1): 1-15. doi: 10.1002/biof.5520310101
- Jariwalla R, Gangapurkar B, Pandit A, Kalinovsky T, Niedzwiecki A, Rath M. Micronutrient Cooperation in Suppression of HIV Production in Chronically and Latently Infected Cells. Mol Med 2010; 3(3): 377-85. doi: 10.3892/mmr_00000268.
- Deryabin PG, Lvov DK, Botikov AG, et al. Effects of a nutrient mixture on infectious properties of the highly pathogenic strain of avian influenza virus A/H5N1. Biofactors. 2008; 33(2): 85-97. doi: 10.1002/biof.5520330201.
- Barbour EK, Rayya EG, Shaib H, et al. Alleviation of histopathological effects of avian influenza virus by a specific nutrient synergy. International J Appl Res Vet Med. 2007; 5(1): 9-16.
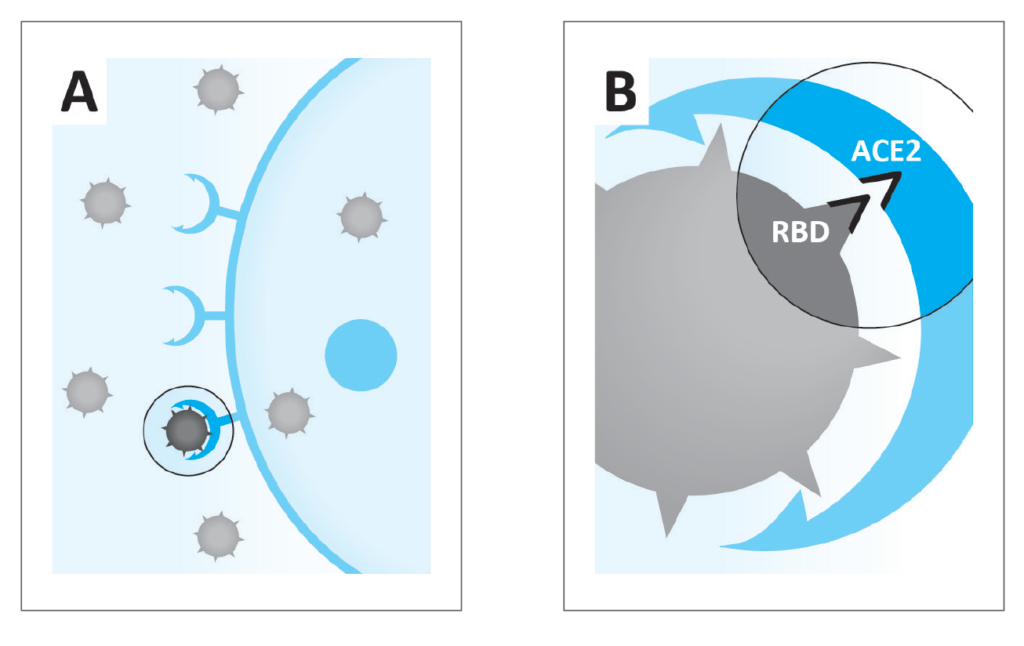 Figure 1: A. A. Coronaviruses infect human body cell via the ACE2 receptor.
Figure 1: A. A. Coronaviruses infect human body cell via the ACE2 receptor.
B. Principle of the commercially available research kit used in this study to test the efficacy of micronutrients combination in blocking attachment of the receptor binding domain (RBD) of the SARS-CoV-2 to the ACE2 receptor on the cell surface

Figure 2: Effects of micronutrient combination on ACE2 receptor expression in human small alveolar epithelial cells. Changes in ACE2 expression are presented as % of control.

Figure 3: Effects of micronutrient combination on ACE2 receptor expression in human small alveolar epithelial cells. Changes in ACE2 expression are presented as % of control.
References
- Chakraborty I, Maity COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci Total Environ. 2020; 728: 138882. https://dx.doi.org/10.1016%2Fj.scitotenv.2020.138882
- WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/. Updated August 13, Accessed August 14, 2020.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020; 382(8): 727-733. doi: 1056/NEJMoa2001017
- Zhou P, Yang XG, Hu B, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579(7798): 270–273. doi: 10.1038/s41586-020-2012-7
- Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature Commun. 2020; 11(1): 1620. doi: 10.1038/ s41467-020-15562-9
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020; 181(2):271-280.e8 doi: 10.1016/j.cell.2020.02.052
- Li Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016; 3(1): 237-261. doi:10.1146/annurev-virology-110615-042301
- Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl Acad. Sci. USA. 2005; 102(22):7988– 7993. https://doi.org/10.1073/pnas.0409465102
- Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The Spike protein of SARS-CoV—A target for vaccine and therapeutic. Nat. Rev. Microbiol. 2009; 7, 226–236. https://doi.org/10.1038/nrmicro2090
- Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang. MERS-CoV Spike protein: a key target for antivirals. Expert Opin Ther Targets. 2017; 21(2): 131-143. doi:10.1080/14728222.2017.1271415
- Ivanov V, Ivanova S, Niedzwiecki A, Rath M. Effective and save global public health strategy to fight the COVID-19 pandemic: Specific micronutrient combination inhibits Coronavirus cell-entry receptor (ACE2) expression. J Cell Med & Nat. Health, 2020.
- Jariwalla RJ, Roomi MW, Gangapurkar B, Kalinovsky T, Niedzwiecki A, Rath M. Suppression of influenza A virus nuclear antigen production and neuraminidase activity by a nutrient mixture containing ascorbic acid, green tea extract and amino acids. 2007; 31(1): 1-15. doi: 10.1002/biof.5520310101
- Jariwalla R, Gangapurkar B, Pandit A, Kalinovsky T, Niedzwiecki A, Rath M. Micronutrient Cooperation in Suppression of HIV Production in Chronically and Latently Infected Cells. Mol Med 2010; 3(3): 377-85. doi: 10.3892/mmr_00000268.
- Deryabin PG, Lvov DK, Botikov AG, et al. Effects of a nutrient mixture on infectious properties of the highly pathogenic strain of avian influenza virus A/H5N1. Biofactors. 2008; 33(2): 85-97. doi: 10.1002/biof.5520330201.
- Barbour EK, Rayya EG, Shaib H, et al. Alleviation of histopathological effects of avian influenza virus by a specific nutrient synergy. International J Appl Res Vet Med. 2007; 5(1): 9-16.

