Aluminum toxicity in Alzheimer‘s disease, breast cancer and vaccine adjuvants
We live in the age of aluminum (Al), faced with an increased exposure to this non-essential metal.1 While Al is ubiquitously present in the earth’s crust, it was not identified until 1808, and was only isolated in pure form in 1825. In 1886 the industrial process of smelting Al was developed (the Hall-Héroulth process), which is still the state-of-the-art technique for Al production. With the onset of World War II, Al became a key strategic metal.2
Today Al and Al compounds are widely present in our lives. As packaging material, Al comes into direct contact with our food and drinks. As an ingredient in cosmetic products it is applied directly to the skin. As part of different pharmaceutical treatments and vaccine adjuvants, it may be taken orally or delivered by intramuscular or subcutaneous injection. In some regions it is even used as coagulant in drinking-water treatment. Given its widespread use, Al can even be found in human breast milk, albeit in lower amounts than in formula milks, especially soya.1,3
So far, no biological function has been identified for this pervasive metal in human and animal organisms, and yet, more and more studies indicate that Al is involved in the development of neurodegenerative diseases (ND) such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS) and amyotrophic lateral sclerosis4 (ALS), while Al-containing adjuvants used in vaccines are believed to be involved in the development of a syndrome not yet recognized by the Food and Drug Administration (FDA): autoimmune/auto-inflammatory syndrome induced by adjuvants (ASIA).5 In addition, it has been suggested that Al plays a role in the increasing prevalence of breast cancer in the upper outside of the breast as a direct consequence of the widespread use of Al-containing antiperspirants.6 But is there hard evidence that Al-toxicity is, indeed, involved in all these pathologies? This paper reviews both the controversy and the findings concerning Al toxicity, the effect of Al and Al-compounds on the human body, and the mechanisms – identified in vitro and in vivo – underlying Al-toxicity.
Introduction
We live in the age of aluminum (Al), faced with an increased exposure to this non-essential metal.1 While Al is ubiquitously present in the earth’s crust, it was not identified until 1808, and was only isolated in pure form in 1825. In 1886 the industrial process of smelting Al was developed (the Hall-Héroulth process), which is still the state-of-the-art technique for Al production. With the onset of World War II, Al became a key strategic metal.2
Today Al and Al compounds are widely present in our lives. As packaging material, Al comes into direct contact with our food and drinks. As an ingredient in cosmetic products it is applied directly to the skin. As part of different pharmaceutical treatments and vaccine adjuvants, it may be taken orally or delivered by intramuscular or subcutaneous injection. In some regions it is even used as coagulant in drinking-water treatment. Given its widespread use, Al can even be found in human breast milk, albeit in lower amounts than in formula milks, especially soya.1,3
So far, no biological function has been identified for this pervasive metal in human and animal organisms, and yet, more and more studies indicate that Al is involved in the development of neurodegenerative diseases (ND) such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS) and amyotrophic lateral sclerosis4 (ALS), while Al-containing adjuvants used in vaccines are believed to be involved in the development of a syndrome not yet recognized by the Food and Drug Administration (FDA): autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA).5 In addition, it has been suggested that Al plays a role in the increasing prevalence of breast cancer in the upper outside of the breast as a direct consequence of the widespread use of Al-containing antiperspirants.6 But is there hard evidence that Al-toxicity is, indeed, involved in all these pathologies? This paper reviews both the controversy and the findings concerning Al toxicity, the effect of Al and Al-compounds on the human body, and the mechanisms – identified in vitro and in vivo – underlying Al-toxicity.
History of the controversy about toxicity of Al
The hypothesis that Al plays a role in the etiology of AD was first raised in 19112, and was posited again in 19657, based on the finding that Al salts injected into rabbits’ brains induced cognitive deficits, in correlation with the accumulation of neurofibrillary changes similar to neurofibrillary tangles (NT) observed in the brains of AD patients. Secondly, Al concentrations were found to be elevated in the brains of AD patients and of patients suffering from dialysis-associated encephalopathy (DAE), a disorder characterized by profound dementia.
However, in the ensuing years, several investigators have contradicted these findings by showing that the neurofibrillary structures in rabbit brains have a different biochemical composition from human neurofibrillary tangles7, and by demonstrating that no AD-like morphology could be identified in the brains of patients with DAE following long-term haemodialysis.8
In addition, it is even questioned whether, in normal circumstances, Al is actually capable of entering the brain and triggering the formation of senile amyloid plaques (SAP), neurofibrillary tangles and neuron degeneration, or whether the blood-brain barrier (BBB) becomes permeable for Al solely as a consequence of the degeneration of neurons by SAP and NT.
Hence, there is still no consensus as to whether or not Al plays a significant role in the etiology of various pathologies, including AD.
In vitro and in vivo observed effect of Al on various biological processes
In vitro and in vivo studies can help us to understand possible mechanisms underlying Al-toxicity in humans.
Affinity of Al to biological structures in animal cells, membranes and extracellular proteins
Since Al has no known biological function in the human body, no specific Al channel or transport mechanism has so far been identified. Though there are many unresolved questions concerning Al transport, the repeatedly identified intracellular presence of Al9 indicates that a form of Al transport must exist.
In general Al is able to compete with other cations, such as calcium (Ca2+), magnesium (Mg2+), zinc (Zn2+), and, in a special way, with Iron (Fe2+/3+). As the Al-cation has only one oxidation state (Al3+), it does not oxidize or reduce, but it can form complexes with other metals. As such, it can also promote oxidative damage by binding to pro-oxidant metals such as iron and copper, thereby creating metal-based oxidative events.10 One example of the toxic effect of Al is the liberation of iron (Fe) from the transferrin complex by Al. The free iron is able to generate highly reactive oxygen species (ROS) via Fenton chemistry. It is believed that free Fe could be the principal mediator of oxidation in cellular systems.2
Al inhibits many Mg2+– and ATP-dependent enzymes, including tubulin GTPase, Na+/K+-ATPase, hexokinase, RNA polymerase, choline acetyltransferase, ferroxidase and calmodulin-dependent ATPase. It binds ATP in a complex that is several orders of magnitude more stable than that with Mg2+.
Fluoroaluminates (AlF-x) complexes are well known to interfere with the activity of G-proteins and calcium homeostasis. It is absorbed in the gastrointestinal (GI) tract and can cross the BBB easily, as shown in rats.2,11 Further, intracellular aluminum chloride (AlCl3) was found to be accompanied by a significant increase of lipid peroxidation (LPO) and membrane viscosity.12
In addition, it has been demonstrated that Al affects chromatin structure; it binds to phosphonucleotides and can alter DNA interaction with nucleoproteins, transcription factors and RNA-polymerase. Some studies show that Al triggers ROS-sensitive transcription factors such as hypoxia-inducible factor-1α (HIF-1α) and nuclear transcription factor -κB (NF-κB).2,11 Both transcription factors play an important role in the development of an inflammatory environment.
Influence on mitochondrial energy production
In vitro studies have indicated that Al toxicity leads to mitochondrial dysfunction. Mitochondrial function is strictly linked to Fe. Hence, by competing with Fe, Al can easily disrupt mitochondrial function. For example, previous studies have shown that the expression of aconitase (ACN), an enzyme that catalyzes isomerization of citrate to isocitrate, is reduced in the presence of Al. The Al-mediated increase of oxidative stress and the Al-associated dysfunction of Fe-dependent enzymes such as ACN perturbs the tricarboxylic acid cycle (TCA) and disables the aerobic ATP production.11 Both oxidative stress and reduced ACN activity are associated with AD, other neurodegenerative pathologies and obesity.
Furthermore, an Al-mediated decrease of L-carnitine was observed, which has been linked to lipid accumulation in hepatic cells12. A recent study in human peripheral blood lymphocytes has confirmed Al-toxicity, but the increase of mitochondrial death and ROS was only significant in higher Al concentrations. Changes in the mitochondrial membrane depolarization and viscosity may depend on AlCl3.12,13
Al increases ROS
The exposure to Al is also associated with the generation of ROS and induction of oxidative stress. On top of that, it decreases endogenous production of super oxide dismutase (SOD) and glutathione (GSH), which can prevent cells from being damaged by ROS.2, 11
The passage of Al through the Blood-Brain-Barrier
Al was found in all body fluids, including the interstitial fluid of the brain. It may cross the BBB via five routes that do not require alterations of the BBB, namely the paracellular, transcellular, active transport, channels and adsorptive or receptor- mediated transport route (fig.1). Once inside the brain, Al persists for a long time, up to 150 days.2 The five mechanisms presented in figure 1 occur in a healthy BBB, whereby Al is transported through the cells. In addition, Al can induce BBB permeability by reducing the number of tight junctions between epithelial cells. As a consequence, Al and other substances that normally cannot cross the BBB, can enter the brain now via this self-induced paracellular route.13 A recent study in Wistar rats indicated that rats exposed to low levels of AlCl3 in the drinking water, have a significantly higher Al brain level than the control group.14
Association between Al toxicity and neurodegenerative pathologies
Alzheimer’s disease
It is generally accepted that Al is a neurotoxic agent, but the exact role of Al and the amount of Al necessary to induce pathology, including neurodegenerative diseases, is not clearly defined. Most studies concerning the association between Al and neuro-toxicology have focused on AD.
An important causal factor of neurodegenerative disease, including AD, is the aforementioned increase of ROS and ROS-induced neuronal cell damage. In addition, neurodegeneration, as seen in AD, PD, ALS and MS, is accompanied by a higher expression of pro-inflammatory genes and concentration of pro-inflammatory cytokines.4
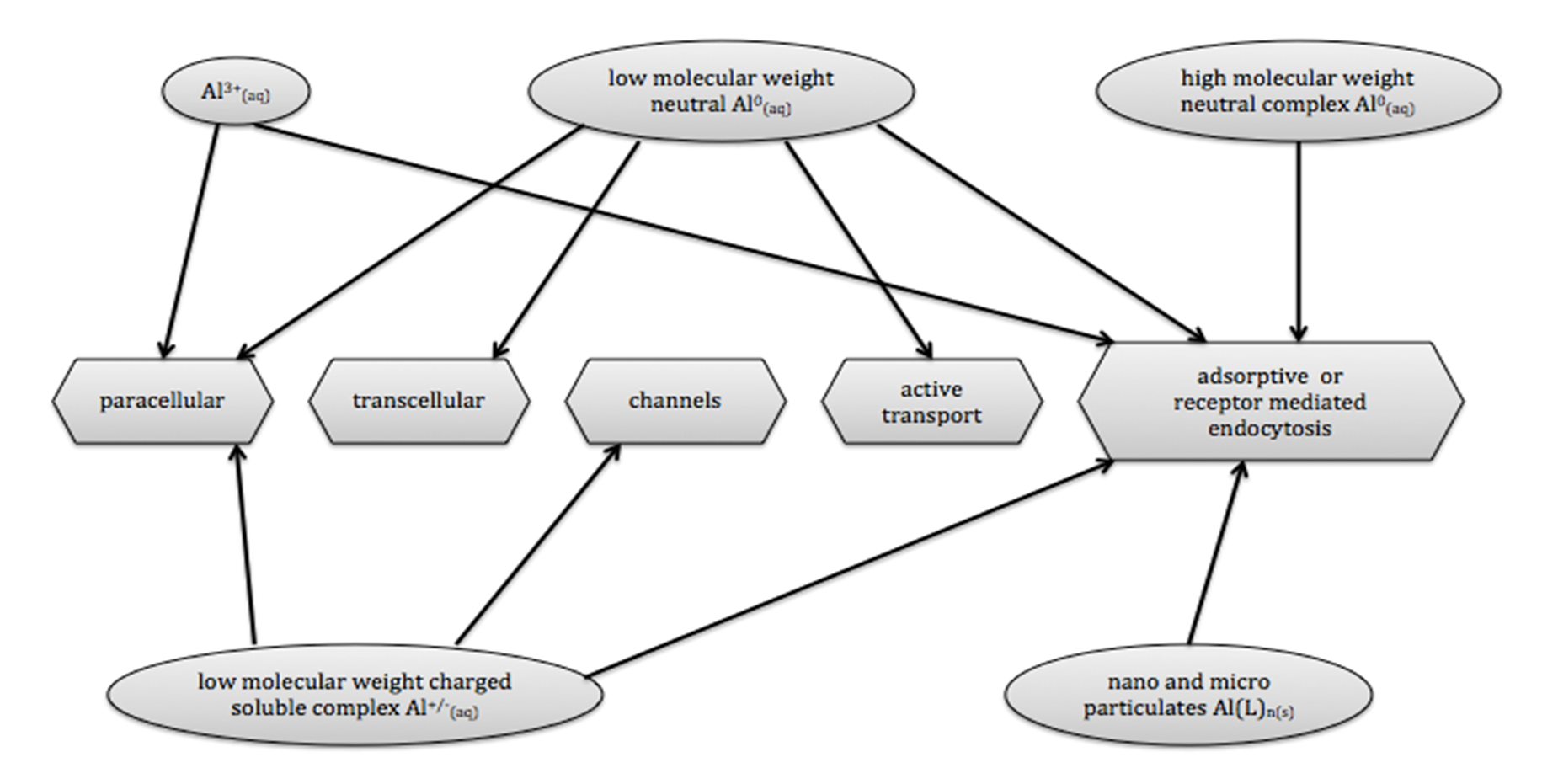
Figure 1 – Routes used by Al-compounds to cross cell membrane and BBB. Solely the verified pathways for the different Al-compounds are presented.9
Also, as previously mentioned, Al increases the expression of NF-κB, which is part of a pro-inflammatory pathway. Moreover, it triggers the formation of Aβ1-42 and NT.
Epidemiological studies
Epidemiological studies have shown that the incidence of AD is higher in those areas where Al concentration in drinking water is relatively high, which strongly suggests that Al exposure is associated with increased risk of AD. 15 Individuals chronically exposed to Al are 71 percent more likely to develop AD. In the light of the aforesaid, it is not surprising that the worldwide increased exposure to Al is accompanied by an increase of AD prevalence. According to the World Alzheimer Report, in 2015 there were 46.8 million people living with dementia. It is predicted that, by 2030 that number will have risen to 74.7 million.16
Human and animal studies
Reusche et al (2001) studied the morphology of post-mortem brains of patients with DAE. DAE is a result of a history of long-term haemodialysis and intake of aluminum-containing drugs. The study indicated that DAE morphology does not match AD morphology. As such, it is one of the studies that seem to discard the AD-Al hypothesis.8
In contrast, a recent study shows that Al is involved in NT formation and growth. Al was found to induce tau-hyperphosphorylation by inhibiting protein phosphatase 2A(PP2A) activity in pyramidal cells.17 Another recent study showed that chronic exposure to drinking water enriched with AlCl3 led to overexpression of amyloid precursor protein (APP) in brains of rats.
With a new method of aluminum-selective fluorescence microscopy, high Al concentration could be detected in human brain tissue of persons with familial AD (the genetic form of AD).18 The authors concluded that brain aluminum may contribute to the development of all forms of AD, at least under certain conditions.19
Lowering the Al burden
Another means to investigate the relationship between Al and AD is by the examination of the impact of decreasing the Al body burden. Hesperidin, a citrus bioflavonoid used to that purpose, had indeed a neuroprotective effect in Wistar rats. Compared with the control group with the same AlCl3 intake14, rats exposed to hesperidin showed a notable reduction of Al levels and acetylcholine esterase (AChE) activity. Moreover, they suffered from less memory loss and fewer histopathological changes in relation to the control group.
In a human study, it was tested whether silicon-rich mineral water can be applied as a non-invasive approach to lower the body Al burden. Fifteen participants consumed a liter of silicon-rich mineral water per day for 12 weeks. After 12 weeks, three of the 15 participants with AD showed a clinically relevant improvement on the Alzheimer’s disease assessment scale-cognitive subscale (ADAS-cog), used to measure cognitive performance.20 A longer-term trial with silicon-rich mineral water is needed to determine if Al is one of the key factors in multifactorial spontaneous AD.
Breast cancer
Another topic related to Al is the possible link between breast cancer and cosmetic products that contain Al compounds. Like AD, breast cancer is a multifactorial disease. In recent years an increase of incidence, especially in the upper outer quadrant of the breast (>50 percent), has been registered. This is a region with more epithelial tissue, located next to the zone were antiperspirant products containing Al are applied.6 Al was found to negatively affect epithelial cells of the breast.6 Higher Al concentrations were detected in breast cancer patients than in non-affected women, and higher Al levels were observed in malignant breast tissue than in adjacent breast tissue.14 However, these findings could not be repeated systematically.21 One of the latest case control studies into the use of underarm cosmetics products (UCP) and the risk of breast cancer concluded that there is a significant increase in the risk of breast cancer, OR=3.88 (95% CI 1.03-14.66, p=0.036) in woman who use UCPs several times per day, starting before age 30.22
Nonetheless, the hypothesized link between breast cancer and Al is still controversial. No mutagenic activity was observed in an Ames test with Al2O3.23 In addition, it is unclear whether the amount of Al absorbed by the skin is high enough to play a role in cancer development. Some studies have showed affinity of Al to estrogen receptors, but others could not confirm this link. The most-used Al compounds in antiperspirants are AlCl3, Al-chlorohydrate or Al-zirconium chlorohydrate glycine complex. The FDA and the European Union (EU) warn against the application of Al-containing products to damaged or irritated skin.24 The absorption of Al via affected skin is more than six times higher, up to 11.5 μg/cm2, compared with 1.81 μg/cm2, in healthy skin.
In vitro studies and possible mechanism that links Al to breast cancer
In immortalized non-transformed human breast epithelial cells exposed to Al salts in concentrations comparable to that in human breast tissues, a significant reduction of DNA-repairing mechanism was observed. In addition, Al was shown to increase DNA double strand break (DSB) in epithelial breast cells. DNA damage and down-regulation of repairing mechanism by Al could induce genome instability, one of the preconditions for cancer development.6
Another in vitro study with estrogen-unresponsive human breast cancer cells showed that Al can disrupt matrix metalloproteinase MMP9 and MMP14 secretion.25 This indicates that Al can increase migration of human breast cancer cells. In a study with human mammary epithelial cells, an increase of DNA synthesis, DSBs and senescence in proliferating cultures was observed. Similar to an activated oncogene, Al triggers senescence and proliferative stress in normal mammary epithelial cells.26
The in vitro studies do not provide evidence that Al is a breast carcinogen, but do reinforce the hypothesis that there is a possible link between the increase of breast cancer and the Al-salts in antiperspirants.
Immune system and Al vaccine adjuvants
Since 1926, Al-salts have been used as adjuvants in vaccines to stimulate the immune system. Vaccine adjuvants containing Al are generally declared as safe. However, this assumption is increasingly questioned.27 Recently, a critical analysis was published of the three toxicokinetics reference studies that are generally used to prove that Al-based adjuvants are harmless. The authors conclude that the sole experimental study examining Al adjuvant kinetics used an inappropriate design, and that the three existent toxicokinetic studies offer insufficient bases to guarantee the absolute safety of Al-adjuvants administrated at large scale.28
Some scientists conclude that Al adjuvants are capable of triggering the immune response even in the absence of a viral or bacterial threat.29 Al induces a release of inflammatory cytokines and matrix metalloproteinase in human monocytes.30 It has been indicated that non-adjuvant Al can induce autoimmune disease and it is plausible that Al from adjuvants may do the same.30
Autoimmune/Auto-inflammatory Syndrome Induced by Adjuvants (ASIA syndrome)
ASIA syndrome, characterized by an abnormal autoimmune response, was recently identified by Shoenfeld and Agmon-Levin. ASIA syndrome incorporates four “enigmatic” medical conditions, Gulf War syndrome, macrophagic myofasciitis (MMF), siliconosis and post-vaccination phenomena, all associated with previous exposure to adjuvants. The link between adjuvants and these four conditions was validated in animal models.27
Using a mouse model, it has been shown that Al is highly bio-persistent. Given the pro-inflammatory effect of Al, this may trigger the development of autoimmune diseases.
A disease pattern such as ASIA syndrome was observed in sheep after a bluetongue vaccine campaign in Spain. A single dose of the ovine vaccine contains 4 mg of Al3+ (the limit of Al for humans is 0.85 mg in the states, 1.25 mg in Europe). So-called “ovine ASIA syndrome” appears in two phases, the acute phase with early manifestations of acute nervous signs, and the chronic phase, characterized by, among other things, the thickening of periphery nerves, necrosis and neuronal loss in the spinal cord.31 The acute phase is not a necessary precondition for the chronic phase to arise.
Mice treated with the aluminum-based adjuvant alum developed inflammation of the submandibular salivary glands and showed higher anti-nuclear antibodies reactivity.
Al-adjuvants and MMF
MMF is a local histopathological reaction in the human deltoid muscle, associated with long-term persistence of vaccine-derived Al-hydroxide (Al(OH)3). It affects mostly women (>70 percent) and can develop up to eight to ten years after vaccination. The majority of people affected with MMF received intramuscular Al injections in the ten years prior to being diagnosed. Clinical manifestations of MMF are diffuse myalgia, arthralgia, chronic fatigue, muscle weakness and cognitive dysfunction.5
Conclusion
Increase of Al in our environment: Is there a need to act?
As mentioned in the introduction, we are living in the Al-age. Al can be found in new places and in compounds that did not previously exist. Until now, no biochemical function of Al could be identified, either in humans or in animals.1 Since its industrial introduction as metal in the Second World War, Al has become an increasingly important material. It is used for construction, in food packaging, as a component of baking formulas and pesticides (Al-phosphide). Aluminum foil that comes in contact with food, particularly acidic or spicy food, will start to dissolve and leach into it. Further, we can find Al in pharmaceutical products, in vaccines and in many other applications.1 In the preparation of drinking water Al-salts are used as flocculants. Owing to the massive use of Al in the different areas of modern society, the human Al body burden is increasing dramatically.1 As a direct consequence, at present Al can even be found in human breast milk (as it can in soy formulas for babies).
Some studies have identified biological mechanisms and pathways responsible for Al toxicity in the human and animal body. However, study results are often contradictory. There is no strong evidence for the association between Al toxicity and the development of pathology. Several studies clearly suggest that Al may affect the microenvironment of, for instance, breast tissue, and stimulate the inflammatory response and oxidative stress, but there is no hard evidence for its role in the etiology of specific pathologies. Hence, there is no conclusive answer for the Al-toxicity hypothesis. Nonetheless, while research continues, it would be wise to do what we can to reduce the aluminum burden by avoiding antiperspirants, aluminum cookware, soft-drink cans, aluminum-containing drugs and the use of Al-adjuvants in vaccines.
References
- Exley C. Human exposure to aluminium. Environ Sci Processes Impacts. 2013; 15(10): 1807-1816.
- Tomeljenovic L. Aluminum and Alzheimer’s disease: after a century of controversy, is there a plausible link? J Alzheimers Dis. 2011; 23: 567-598.
- Fanni D, Ambu R, Gerosa C, et al. Aluminum exposure and toxicity in neonates: a practical guide to halt aluminum overload in the prenatal and perinatal periods. World J Pediatr. 2014; 10(2): 101-107.
- Bondy S. Prolonged exposure to low levels of aluminum leads to changes associated with brain aging and neurodegeneration. Toxicology. 2014; 315: 1-7.
- Shaw C, Li D, Tomljenovic L. Are there negative CNS impacts of aluminum adjuvants used in vaccines and immunotherapy? Immunotherapy. 2014; 6(10): 1055-1071.
- Farasani A, Darbre P. Effects of aluminium chloride and aluminium chlorohydrate on DNA repair in MCF10A immortalised non-transformed human breast epithelial cells. J Inorg Biochem. 2015; 152: 186-189.
- Lidsky T. Is the Aluminum Hypothesis Dead? J Occup Environ Med. 2014; 56(5 Suppl): S73-S79.
- Reusche E, Koch V, Lindner B, Harrison A, Friedrich H. Alzheimer morphology is not increased in dialysis-associated encephalopathy and long-term hemodialysis. Acta Neuropathol. 2001; 101(3): 211-6.
- Exley C, Mold M. The binding, transport and fate of aluminium in biological cells. J Trace Elem Med Biol. 2015; 30: 90-95.
- Kumar V, Gill K. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: A review. Neurotoxicology. 2014; 41:154-166.
- Mailloux R, Lemire J, Appanna V. Hepatic response to aluminum toxicity: Dyslipidemia and liver diseases. Exp Cell Res. 2011; 317(16): 2231-2238.
- Skarabahatava A, Lukyanenko L, Slobozhanina E, et al. Plasma and mitochondrial membrane perturbation induced by aluminum in human peripheral blood lymphocytes. J Trace Elem Med Biol. 2015; 31: 37-44.
- Çabuş N, Oğuz E, Tufan A, Adıgüzel E. A histological study of toxic effects of aluminium sulfate on rat hippocampus. Biotech Histochem. 2014; 90(2): 132-139.
- Darbre P, Mannello F, Exley C. Aluminium and breast cancer: Sources of exposure, tissue measurements and mechanisms of toxicological actions on breast biology. J Inorg Biochem. 2013; 128: 257-261.
- Wang Z, Wei X, Yang J, et al. Chronic exposure to aluminum and risk of Alzheimer’s disease: A meta-analysis. Neurosci Lett. 2016; 610: 200-206.
- Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. World Alzheimer Report. The Global Impact of Dementia. An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International. Published August, 2015. Republished with corrections October, 2015. www.alz.co.uk/research/WorldAlzheimerReport2015.pdf Accessed May 10, 2016.
- Walton J. Evidence for Participation of Aluminum in Neurofibrillary Tangle Formation and Growth in Alzheimer’s Disease. J Alzheimers Dis. 2010; 22(1): 65-72.
- Mirza A, King A, Troakes C, Exley C. Aluminium in brain tissue in familial Alzheimer’s disease. J Trace Elem Med Biol. 2017; 40: 30-36.
- Exley C. Why Industry Propaganda and Political Interference Cannot Disguise the Inevitable Role Played by Human Exposure to Aluminum in Neurodegenerative Diseases, Including Alzheimer’s Disease. Front Neurol. 2014; 5.
- Davenward S, Bentham P, Wright J, et al. Silicon-Rich Mineral Water as a Non-Invasive Test of the ‘Aluminium Hypothesis’ in Alzheimer’s Disease. J Alzheimers Dis. 2013; 33(2): 423-430.
- Rodrigues-Peres R, Cadore S, Febraio S, et al. Aluminum concentrations in central and peripheral areas of malignant breast lesions do not differ from those in normal breast tissues. BMC Cancer. 2013; 13(1): 104.
- Linhart C, Talasz H, Morandi E, et al. Use of Underarm Cosmetic Products in Relation to Risk of Breast Cancer: A Case-Control Study. EBioMedicine. 2017; 21: 79-85.
- Balasubramanyam A, Sailaja N, Mahboob M, Rahman M, Hussain S, Grover P. In vitro mutagenicity assessment of aluminium oxide nanomaterials using the Salmonella/microsome assay. Toxicol In Vitro. 2010; 24(6): 1871-1876.
- REGULATION (EC) No 1223/2009 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 30 November 2009 on cosmetic products. Official Journal of the European Union. December 22, 2009. www.ec.europa.eu/health/endocrine_disruptors/docs/cosmetic_1223_2009_regulation_en.pdf Accessed May 27, 2016.
- Bakir A, Darbre P. Effect of aluminium on migration of oestrogen unresponsive MDA-MB-231 human breast cancer cells in culture. J Inorg Biochem. 2015; 152: 180-185.
- Sappino A, Buser R, Lesne L, et al. Aluminium chloride promotes anchorage-independent growth in human mammary epithelial cells. J Appl Toxicol. 2012; 32(3): 233-243.
- Butnaru D, Shoenfeld Y. Adjuvants and lymphoma risk as part of the ASIA spectrum. Immunol Res. 2015; 61(1-2): 79-89.
- Masson J, Crépeaux G, Authier F, Exley C, Gherardi R. Critical analysis of reference studies on the toxicokinetics of aluminum-based adjuvants. J Inorg Biochem. 2018; 181: 87-95.
- Shaw C, Tomljenovic L. Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res. 2013; 56(2-3): 304-316.
- Ligi D, Santi M, Croce L, Mannello F. Aluminum induces inflammatory and proteolytic alterations in human monocytic cell line. J Inorg Biochem. 2015; 152: 190-198.
- Luján L, Pérez M, Salazar E, Álvarez N, Gimeno M, Pinczowski P et al. Autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA syndrome) in commercial sheep. Immunol Res. 2013; 56(2-3): 317-324.

Figure 1 – Routes used by Al-compounds to cross cell membrane and BBB. Solely the verified pathways for the different Al-compounds are presented.9
- Exley C. Human exposure to aluminium. Environ Sci Processes Impacts. 2013; 15(10): 1807-1816.
- Tomeljenovic L. Aluminum and Alzheimer’s disease: after a century of controversy, is there a plausible link? J Alzheimers Dis. 2011; 23: 567-598.
- Fanni D, Ambu R, Gerosa C, et al. Aluminum exposure and toxicity in neonates: a practical guide to halt aluminum overload in the prenatal and perinatal periods. World J Pediatr. 2014; 10(2): 101-107.
- Bondy S. Prolonged exposure to low levels of aluminum leads to changes associated with brain aging and neurodegeneration. Toxicology. 2014; 315: 1-7.
- Shaw C, Li D, Tomljenovic L. Are there negative CNS impacts of aluminum adjuvants used in vaccines and immunotherapy? Immunotherapy. 2014; 6(10): 1055-1071.
- Farasani A, Darbre P. Effects of aluminium chloride and aluminium chlorohydrate on DNA repair in MCF10A immortalised non-transformed human breast epithelial cells. J Inorg Biochem. 2015; 152: 186-189.
- Lidsky T. Is the Aluminum Hypothesis Dead? J Occup Environ Med. 2014; 56(5 Suppl): S73-S79.
- Reusche E, Koch V, Lindner B, Harrison A, Friedrich H. Alzheimer morphology is not increased in dialysis-associated encephalopathy and long-term hemodialysis. Acta Neuropathol. 2001; 101(3): 211-6.
- Exley C, Mold M. The binding, transport and fate of aluminium in biological cells. J Trace Elem Med Biol. 2015; 30: 90-95.
- Kumar V, Gill K. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: A review. Neurotoxicology. 2014; 41:154-166.
- Mailloux R, Lemire J, Appanna V. Hepatic response to aluminum toxicity: Dyslipidemia and liver diseases. Exp Cell Res. 2011; 317(16): 2231-2238.
- Skarabahatava A, Lukyanenko L, Slobozhanina E, et al. Plasma and mitochondrial membrane perturbation induced by aluminum in human peripheral blood lymphocytes. J Trace Elem Med Biol. 2015; 31: 37-44.
- Çabuş N, Oğuz E, Tufan A, Adıgüzel E. A histological study of toxic effects of aluminium sulfate on rat hippocampus. Biotech Histochem. 2014; 90(2): 132-139.
- Darbre P, Mannello F, Exley C. Aluminium and breast cancer: Sources of exposure, tissue measurements and mechanisms of toxicological actions on breast biology. J Inorg Biochem. 2013; 128: 257-261.
- Wang Z, Wei X, Yang J, et al. Chronic exposure to aluminum and risk of Alzheimer’s disease: A meta-analysis. Neurosci Lett. 2016; 610: 200-206.
- Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. World Alzheimer Report. The Global Impact of Dementia. An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International. Published August, 2015. Republished with corrections October, 2015. www.alz.co.uk/research/WorldAlzheimerReport2015.pdf Accessed May 10, 2016.
- Walton J. Evidence for Participation of Aluminum in Neurofibrillary Tangle Formation and Growth in Alzheimer’s Disease. J Alzheimers Dis. 2010; 22(1): 65-72.
- Mirza A, King A, Troakes C, Exley C. Aluminium in brain tissue in familial Alzheimer’s disease. J Trace Elem Med Biol. 2017; 40: 30-36.
- Exley C. Why Industry Propaganda and Political Interference Cannot Disguise the Inevitable Role Played by Human Exposure to Aluminum in Neurodegenerative Diseases, Including Alzheimer’s Disease. Front Neurol. 2014; 5.
- Davenward S, Bentham P, Wright J, et al. Silicon-Rich Mineral Water as a Non-Invasive Test of the ‘Aluminium Hypothesis’ in Alzheimer’s Disease. J Alzheimers Dis. 2013; 33(2): 423-430.
- Rodrigues-Peres R, Cadore S, Febraio S, et al. Aluminum concentrations in central and peripheral areas of malignant breast lesions do not differ from those in normal breast tissues. BMC Cancer. 2013; 13(1): 104.
- Linhart C, Talasz H, Morandi E, et al. Use of Underarm Cosmetic Products in Relation to Risk of Breast Cancer: A Case-Control Study. EBioMedicine. 2017; 21: 79-85.
- Balasubramanyam A, Sailaja N, Mahboob M, Rahman M, Hussain S, Grover P. In vitro mutagenicity assessment of aluminium oxide nanomaterials using the Salmonella/microsome assay. Toxicol In Vitro. 2010; 24(6): 1871-1876.
- REGULATION (EC) No 1223/2009 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 30 November 2009 on cosmetic products. Official Journal of the European Union. December 22, 2009. www.ec.europa.eu/health/endocrine_disruptors/docs/cosmetic_1223_2009_regulation_en.pdf Accessed May 27, 2016.
- Bakir A, Darbre P. Effect of aluminium on migration of oestrogen unresponsive MDA-MB-231 human breast cancer cells in culture. J Inorg Biochem. 2015; 152: 180-185.
- Sappino A, Buser R, Lesne L, et al. Aluminium chloride promotes anchorage-independent growth in human mammary epithelial cells. J Appl Toxicol. 2012; 32(3): 233-243.
- Butnaru D, Shoenfeld Y. Adjuvants and lymphoma risk as part of the ASIA spectrum. Immunol Res. 2015; 61(1-2): 79-89.
- Masson J, Crépeaux G, Authier F, Exley C, Gherardi R. Critical analysis of reference studies on the toxicokinetics of aluminum-based adjuvants. J Inorg Biochem. 2018; 181: 87-95.
- Shaw C, Tomljenovic L. Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res. 2013; 56(2-3): 304-316.
- Ligi D, Santi M, Croce L, Mannello F. Aluminum induces inflammatory and proteolytic alterations in human monocytic cell line. J Inorg Biochem. 2015; 152: 190-198.
- Luján L, Pérez M, Salazar E, Álvarez N, Gimeno M, Pinczowski P et al. Autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA syndrome) in commercial sheep. Immunol Res. 2013; 56(2-3): 317-324.