Inhibition of tumor growth and metastasis by a novel nutrient mixture of inoculation of mouse mammary 4T1 carcinoma in kidney of female Balb/c mice
Breast cancer is the most prevalent and commonly diagnosed cancer in women worldwide. Though it is treatable in early stages, there is no effective treatment available once it metastasizes. Renal cell carcinoma (RCC) incidence is also on the rise and most of the patients present with metastatic cancer at the time of diagnosis. RCC is frequently presented with lung metastases that are poorly responsive to the conventional treatment of chemotherapy, and hormone and radiation therapy.
We studied the effect of a novel nutrient mixture (NM) containing ascorbic acid, lysine, proline, green tea extract, and quercetin on inhibition of tumor growth and metastasis by inoculation of mouse mammary carcinoma 4T1 cells in the kidney of female BALB/c mice. The 5- to 6-weeks-old BALB/c mice (n=10), were inoculated in the left kidney with 5 x 105 4T1 cells in 100 µl media. The right kidney was left untreated. After injection, the mice were randomly divided into two groups. The control group of mice (n = 5) were fed a regular mouse chow diet and the NM group (n = 5) was given the same diet but supplemented with 0.5% NM. After two weeks the mice were sacrificed and the abdominal cavities were opened. Both kidneys and other organs were excised and examined for metastasis. The left kidney in the control group was significantly enlarged due to 4T1 tumor growth. In contrast, the left kidney in the NM group was less enlarged. Severe lung and spleen metastases were observed in the control group and mild metastasis was seen in the lungs and spleen of the NM group. The right kidney in both the control and NM group was unaffected. Neither group showed any metastatic lesions in the liver or heart. In conclusion, these results suggest that the NM has the potential to suppress tumor growth and tumor metastasis to the lungs and spleen.
Introduction
Worldwide, breast cancer is the most commonly diagnosed cancer in women and has become a topic of rigorous research. It is the second leading cause of cancer death in women in the USA, affecting one in twelve women. According to the American Cancer Society’s estimates, approximately 266,120 new cases of invasive breast cancer and 63,960 new cases of non-invasive (in situ) breast cancer are expected to be diagnosed in 2018 in the USA. Approximately 40,920 women in the USA are expected to die in 2018 from breast cancer.1 The 5-year survival rate of a breast cancer patient is 66 percent when the cancer is detected early. Upon diagnosis, a breast cancer patient undergoes surgery to remove the tumor followed by chemotherapy or radiation therapy. However, there is no effective treatment after it metastasizes to distant organs. Though treatable in early stages, once metastasis has occurred the survival rate of breast cancer is drastically reduced to a median of 2 to 3 years and treatment focuses on palliative care.2 Advanced metastasized breast cancer has a poor outcome. The 5-year survival rate for stage IV breast cancer is 22 percent.
Renal cell carcinoma (RCC) incidence has risen in recent years and most of the RCC patients present with metastatic cancer at the time of diagnosis. RCC is a highly erratic and unpredictable cancer even when diagnosed at an early stage. Except for blood in the urine, other symptoms such as abdominal, back and flank pain, weight loss, and abnormal blood counts are vague signs and delay the diagnosis. RCC is frequently presented with lung metastasis that is poorly responsive to the conventional treatment of chemotherapy, and hormone and radiation therapy. The 5-year survival rate of metastasized kidney cancer patients is 60 percent.
Critical events in tumor metastasis include cell attachment, proteolytic degradation of the extracellular matrix (ECM) and migration through the disrupted matrix.3 The high risk of metastasis makes breast cancer difficult to treat and accounts for an approximately 90 percent mortality due to metastasized cancer.
A major problem in studying metastasis has been the lack of suitable models that faithfully represent the metastatic process as it occurs in vivo. While some human xenograft models can approximate primary tumor growth in mice, replication of tumor metastasis is more problematic.4 Generally, human tumor cells metastasize poorly in mice and metastases are associated with unexpected characteristics. In contrast, murine tumor cell models often metastasize more effectively and display metastatic characteristics more similar to those observed in cancer patients.5 In order to mimic the high potential of tumor metastasis in humans, the mouse mammary cancer 4T1 cells were chosen. The 4T1 cells are highly invasive and metastasize to the lungs, liver, brain, bones, and spleen. This is not surprising since microenvironments and tumor-host interactions play important roles in tumor cell behavior. When introduced orthotopically, 4T1 is capable of metastasizing to several organs including the lungs, liver, brain and the bones.6
We studied the effect of a novel nutrient mixture (NM) containing ascorbic acid, lysine, proline, green tea extract, and quercetin by injecting the mouse mammary tumor 4T1 cells into the left kidney of female BALB/c mice. Our main objective was to determine the effect of dietary supplementation with NM on the development of tumors and metastasis to other organs in mice that had been injected with the 4T1 cells. The 4T1 mammary carcinoma model was chosen as it has several characteristics that make it a suitable experimental animal model for human mammary cancer growth and metastasis.7 The metastatic spread of 4T1 to other organs and the draining lymph nodes is similar to that found in human breast cancer.7
Methods and Materials
Cancer cell lines and culture
The murine breast carcinoma cell line 4T1 was obtained from ATCC (American Type Culture Collection, Rockville, MD, USA). The 4T1 cells were maintained in DMEM supplemented with 10 percent fetal bovine serum, and 100 U/ml penicillin (antibiotic) and 100 μg/ml streptomycin (antibiotic). The media and sera used were obtained from ATCC, and penicillin and streptomycin (antibiotics) were from Gibco BRL (Long Island, NY, USA).
Animals
The Female BALB/c mice (approximately 5 to 6 weeks old on arrival) were purchased from Simonsen Laboratories (Gilroy, CA, USA). They were maintained in microisolator cages under pathogen-free conditions on a 12-h light/12-h dark schedule for one week. All procedures were performed according to humane and customary care and use of experimental animals and followed a protocol approved by the Internal Animal Care and Use Committee (IACUC).
Animal Diet and the composition of Nutrient Mixture
The regular rodent diet was obtained from Purina Mills (Gray Summit, MO, USA). The NM 0.5 percent supplemented diet mix was milled and pressed by Purina Mills, LLC, and generated by Vitatech (Tustin, CA, USA). The NM 0.5 percent diet comprised the following in the ratio indicated: vitamin C (as ascorbic acid and as Mg, Ca, and palmitate ascorbate) 700 mg; L-lysine 1000 mg; L-proline 750 mg; L-arginine 500 mg; N-acetyl cysteine 200 mg; standardized green tea extract (80 percent polyphenol) 1000 mg; quercetin from quercetin dihydrate, saphora japonica 50 mg; selenium 30 µg; copper 2 mg; and manganese 1 mg.
Experimental design
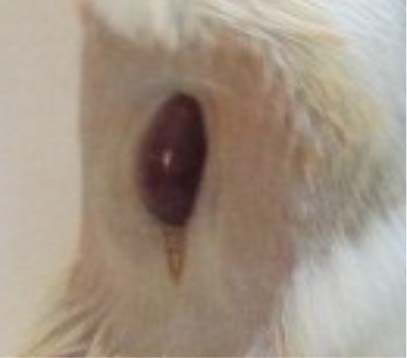
Figure 1 – Left kidney exposed. Orthotopic inoculation of 4T1 cells in the exposed kidney of female BALB/c mouse
After housing for one week, the female BALB/c mice (n = 10) were anesthetized by inhalation of isofluorane USP (Abbott Laboratories, Chicago, IL, USA). The left side of the back was sterilely prepared and an incision of 1 cm was made to expose the left kidney (Figure 1). Using a 26-gauge needle, the mice were injected in the left kidney with 5 x 105 4T1 cells, superficially in 100 µl. The needle was removed slowly from the capsule and the injected portion was immediately blotted over with a sterile cotton pad to ensure complete absorption and to prevent leakage. Finally, the area was sutured and clip-closed. The right kidney was left untreated.
After injection, the mice were randomly divided into two groups and maintained for two weeks on the following diets: the control group (n = 5) mice were fed regular Purina mouse chow and the NM group (n = 5) the regular diet supplemented with 0.5 percent NM (w/w). During the study, the mice consumed, on average, 4 g of their respective diets per day. Thus, the supplemented mice received ~20 mg of 0.5 percent NM per day.
After two weeks the mice were sacrificed, the abdominal cavities were opened and the kidneys, lungs, livers, hearts and spleens were excised from all the animals and examined for tumor growth and metastasis. Growth of 4T1 colonies in the left kidneys was evaluated by sectioned tissue and metastasis to the vital organs was observed. All procedures were performed according to humane and customary care and use of experimental animals and conducted under protocols approved by the IACUC.
Histopathology
The kidney samples were fixed in 10 percent buffered formalin, embedded in paraffin and cut into 4 to 5-micron sections. The sections were deparaffinized through xylene and graduated alcohol series to water, and stained with H&E for microscopic evaluation by IDEXX Reference Laboratories.
Results
Mean initial and final weights of the animals
The body weight of the mice in both the groups did not differ significantly. Prior to the experiment, the animals in the control group weighed 19.01 ± 0.52 gm at the time of inoculation, and 20.3 ± 0.83 gm at the time of sacrifice. Similarly, the animals in the NM group weighed 19.03 ± 0.050 gm before the experiment, and 19.86 ± 1.22 gm at the time of sacrifice.
Tumor morphology
The mouse abdominal cavity was opened and the kidney was excised. The metastasis to other vital organs such as the liver, spleen and the lungs were visually examined. The weight of the kidney in the control group was approximately 25-30 percent more than the kidney in the NM-treated mice. The weight of the left kidney in the control group was 0.49 ± 0.02 gm and in the NM group the weight was 0.38 ± 0.03 gm (significance p < 0.05).

Figure 2A – Organs from control group
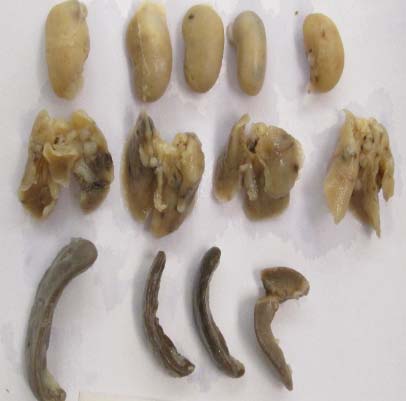
Figure 2B – Organs from NM group
Figure 2 – Visual Comparison of metastatic areas in vital organs (kidney, lungs, spleen) after 4T1 inoculation in BALB/c mice
Metastasis in vital organs
The lungs and the spleen in the control group showed enlargement and multiple metastatic spots as shown in Figure 2A. Tumor metastasis to the lungs was found in 4 out of 5 of the control group mice. However, mild metastasis was observed in the NM-treated group as seen in Figure 2B. There were no metastases found in the liver or heart of either group, and the right kidney was not affected.
Tumor Histopathology
Cross section of the left kidney of female BALB/c mice in the control as well as the NM-treated group is seen in Figures 3A and 3B. Histopathological reporting showed multiple large metastases in the kidney and destruction of significant portions of normal kidney tissue. The tumor in the control group showed large areas of necrosis involving 60 percent of the mass, and metastatic lesions consisting of nests and sheaths of irregularly round cells and spindle-shaped cells with prominent round or oval nuclei and poorly defined cytoplasmic borders, showing significant destruction of normal kidney tissue (Figure 4A and 4B).

Figure 3A – Cross-section of kidney in control group
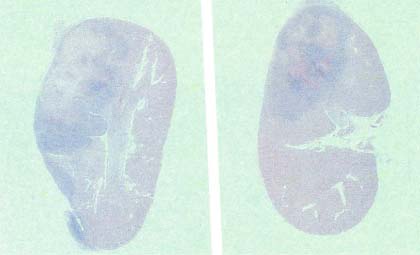
Figure 3B – Cross-section of kidney in NM group
Figure 3 – Colonization and tumor formation of 4T1 cells as seen in the kidney of female BALB/c mice
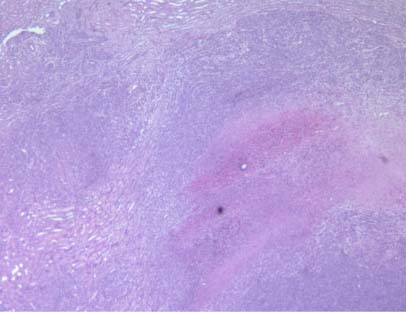
Figure 4A – control group
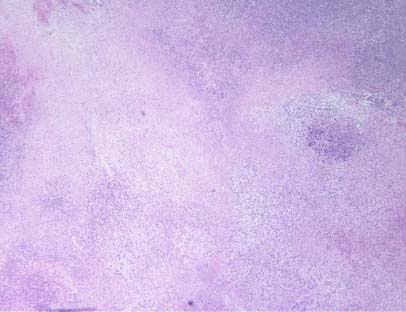
Figure 4B – control group
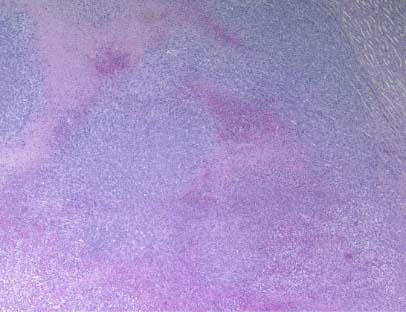
Figure 4C – NM group
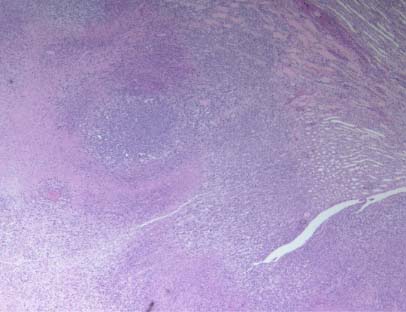
Figure 4D – NM group
Figure 4 – Tumor histopathology in kidney after inoculation of BALB/c female mice with 4T1 mouse mammary carcinoma cells BALB/c
The tumors in the NM group indicated cell morphology of 4T1 cells with smaller areas of necrosis. Most of the kidney in the NM group did not show metastatic changes (Figure 4C and 4D).
Discussion
Our objective in this study was to examine the effect of supplementation with NM on mouse mammary tumor growth and metastasis. The model of injection of 4T1 cells into the kidney of female BALB/c mice led to tumor growth and lung and spleen metastasis in the control group, which indicated success of the model. The results of this in vivo study demonstrated significant decrease in the tumor growth in the kidney and inhibition of metastatic potential in the test group supplemented with NM. Metastasis to the lungs and spleen was significantly reduced by NM supplementation. There was no metastasis to the heart and the liver. Therefore, NM inhibited metastasis to lungs compared to the control group, which exhibited 100 percent lung metastasis.
These results support our previous in vitro and in vivo studies using 4T1 cells injected into the mammary pads, where the NM was able to reduce the tumor burden by 50 percent.6 In that study the metastasis to the lungs, spleen, kidney, heart, and liver were also seen to be significantly reduced with NM supplementation. High levels of the collagenase enzymes matrix metalloproteinases (MMP-2 and MMP-9) have also been found to correlate with the aggressiveness of breast cancer.8,9 Our in vitro studies showed that NM exhibited toxicity on cancer cells in a dose-dependent manner. NM successfully suppressed cell proliferation, secretion of MMP-2 and MMP-9, and migration and Matrigel invasion by 4T1 cells.6 We also compared aggressiveness of the tumor and MMP expression and found that MMP expression was associated with the Matrigel invasion and aggressiveness of the tumor.10
Breast cancer patients often have detectable or occult metastases at diagnosis and most patients will develop metastatic lesions during the course of the disease. We investigated the effect of a nutrient mixture (NM) containing ascorbic acid, lysine, proline, green tea extract and quercetin on murine breast cancer 4T1, a unique metastatic breast cancer model that has the capacity to metastasize efficiently to the same sites as are found in human breast cancer. Tao, et al., reported that metastasis of 4T1 tumors was associated with extensive necrosis and inflammation within the primary tumor and hematopoiesis in several mouse organs including the spleen and liver.7 In our study, both the control and NM supplemented groups demonstrated irregularly round subcutaneous tumors with areas of tumor necrosis. The necrotic areas were more pronounced (60 percent) in the kidneys of the control group of mice than in the NM group.
Bonfil, et al., reported that the distribution of necrosis within a primary tumor was partially responsible for the development of metastases.11 In another study Bonfil, et al., also reported that the tumor necrosis was an important source of gelatinase/type IV collagenase, mainly in its 92-kDa form, and thus played a major role in tumor invasion.12 Our findings correlate with these studies and further demonstrate the efficacy of NM in reducing necrosis and potential for metastasis.
High consumption of fruits and vegetables have been reported to be associated with prevention, inhibition, and reversal of cancers.13,14 We developed strategies to inhibit cancer development and its spread by targeting common mechanisms used by the cancer cells.15
NM formulation was based on addressing critical physiological targets in cancer progression and metastasis. Adequate supplies of ascorbic acid, lysine, and proline are essential to ensuring optimal structure of ECM, and lysine supports ECM stability as a natural inhibitor of plasmin-induced proteolysis.16 The anticancer effects of quercetin include induction of cell cycle arrest and apoptosis.17 In our other studies we have shown that the addition of quercetin significantly increased the anticancer efficacy of green tea extract and reduced mammary tumors in wistar rats.18
We have also demonstrated that injection of 4T1 cells in vitamin C-deficient Gulo KO mice resulted in larger tumors with necrosis and poorly defined borders than the tumors developed in the Gulo KO mice supplemented with ascorbate. The ascorbate- supplemented group of mice had smaller tumors with less necrosis and enhanced collagen encapsulation, signifying less metastatic potential.19
In conclusion, the results of this in vivo study of murine 4T1 cells injected into the kidney of BALB/c mice confirmed the validity of this model to study breast cancer metastasis since, as in human cancer, metastasis was observed in the lungs and spleen. The present study also demonstrated significant suppression of tumor growth and metastasis to the lungs and spleen with NM dietary supplementation. Since current treatment methods for breast cancer are generally ineffective, there is a need for the development of effective therapeutic agents for these cancers with minimal toxicity. Our studies demonstrated that the mixture of the non-toxic components of NM significantly suppressed breast cancer development and metastasis.
Acknowledgments
The research study was funded by Dr. Rath Health Foundation (Santa Clara, CA), a non-profit organisation.
References
- Breast Cancer Survival Rates. American Cancer Society Website. www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html Published September 25, 2017. Updated: December 20, 2017. Accessed April 11, 2018.
- Ali SM, Harvey HA, Lipton A. Metastatic breast cancer: overview of treatment. Clin Orthop. 2003; 414(Suppl): 132–137.
- Fidler IJ. Molecular biology of cancer: invasion and metastasis. In: De Vita VT, Hellman S, Rosenberg SA (eds). Cancer: Principles and Practice of Oncology. 5th ed. Philadelphia, PA: Lippincott-Raven; 1997: 135-152.
- Bibby MC. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer. 2004; 40: 852–857.
- Vernon E, Bakewell SJ, Chodash LA. Deciphering the molecular basis of breast cancer metastasis with mouse models. Rev Endocr Metab Disord. 2007; 8: 199–213.
- Roomi MW, Kalinovsky T, Roomi NW, et al. In vitro and in vivo effects of a nutrient mixture on breast cancer progression. Int J of Oncology. 2014; 44: 1933-1944.
- Tao K, Fang M, Aloy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008; 8: 228.
- Bachmeier BE, Nerlich AG, Lichtinghagen R, Sommerhoff CP. Matrix metalloproteinases (MMPs) in breast cancer cell lines of different tumorigenicity. Anticancer Res. 2001; 21(6A): 3821-3828.
- Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER-2, and prognosis. Clin Cancer Res. 2004; 10(22): 7621-7628.
- Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Inhibition of invasion and MMPs by a nutrient mixture in human cancer cell lines: a correlation study. Exp Oncol. 2010; 32(4): 243-8.
- Bonfil RD, Bustuabad OD, Ruggiero RA, Meiss RP, Pasqualini CD. Tumor necrosis can facilitate the appearance of metastases. Clin Exp Metastasis. 1998; 6(2): 121-129.
- Bonfil RD, Medina PA, Gómez DE, et al. Expression of gelatinase/type IV collagenase in tumor necrosis correlates with cell detachment and tumor invasion. Clin Exp Metastasis. 1992; 10(3): 211-220.
- Aldercreutz H. Western diet and Western disease: some hormonal and biochemical mechanisms and associations. Scand. J. Clin. Lab. Investig. 1990; 50(Suppl. 201): 3–23.
- Miller AB. Diet and Cancer. A review. Acta Oncol. 1990; 29(1): 87–95.
- Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Micronutrient synergy — A new tool in effective control of metastasis and other key mechanisms of cancer. Cancer Metastasis Rev. 2010; 29(3): 529–543.
- Rath M, Pauling L. Plasmin-induced proteolysis and the role of apoprotein(a), lysine and synthetic analogs. Orthomol. Med. 1992; 7(1): 17–23.
- Gibellini L, Pinti M, Nasi M, et al. Quercetin and cancer chemoprevention. Evid. Based Complement. Altern. Med. 2011, Article ID 591356
- Kale A, Gawande S, Kotwal S, et al. A combination of green tea extract, specific nutrient mixture and quercetin: An effective intervention treatment for the regression of N-methyl-N-nitrosourea (MNU)-induced mammary tumors in Wistar rats. Oncol Lett. 2010; 1(2): 313-317.
- Cha J, Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int J Oncol. 2013; 42(1): 55-64.



Figure 2 – Visual Comparison of metastatic areas in vital organs (kidney, lungs, spleen) after 4T1 inoculation in BALB/c mice


Figure 3 – Colonization and tumor formation of 4T1 cells as seen in the kidney of female BALB/c mice

Figure 4A – control group

Figure 4B – control group

Figure 4C – NM group

Figure 4D – NM group
Figure 4 – Tumor histopathology in kidney after inoculation of BALB/c female mice with 4T1 mouse mammary carcinoma cells BALB/c
References
- Breast Cancer Survival Rates. American Cancer Society Website. www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html Published September 25, 2017. Updated: December 20, 2017. Accessed April 11, 2018.
- Ali SM, Harvey HA, Lipton A. Metastatic breast cancer: overview of treatment. Clin Orthop. 2003; 414(Suppl): 132–137.
- Fidler IJ. Molecular biology of cancer: invasion and metastasis. In: De Vita VT, Hellman S, Rosenberg SA (eds). Cancer: Principles and Practice of Oncology. 5th ed. Philadelphia, PA: Lippincott-Raven; 1997: 135-152.
- Bibby MC. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer. 2004; 40: 852–857.
- Vernon E, Bakewell SJ, Chodash LA. Deciphering the molecular basis of breast cancer metastasis with mouse models. Rev Endocr Metab Disord. 2007; 8: 199–213.
- Roomi MW, Kalinovsky T, Roomi NW, et al. In vitro and in vivo effects of a nutrient mixture on breast cancer progression. Int J of Oncology. 2014; 44: 1933-1944.
- Tao K, Fang M, Aloy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008; 8: 228.
- Bachmeier BE, Nerlich AG, Lichtinghagen R, Sommerhoff CP. Matrix metalloproteinases (MMPs) in breast cancer cell lines of different tumorigenicity. Anticancer Res. 2001; 21(6A): 3821-3828.
- Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER-2, and prognosis. Clin Cancer Res. 2004; 10(22): 7621-7628.
- Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Inhibition of invasion and MMPs by a nutrient mixture in human cancer cell lines: a correlation study. Exp Oncol. 2010; 32(4): 243-8.
- Bonfil RD, Bustuabad OD, Ruggiero RA, Meiss RP, Pasqualini CD. Tumor necrosis can facilitate the appearance of metastases. Clin Exp Metastasis. 1998; 6(2): 121-129.
- Bonfil RD, Medina PA, Gómez DE, et al. Expression of gelatinase/type IV collagenase in tumor necrosis correlates with cell detachment and tumor invasion. Clin Exp Metastasis. 1992; 10(3): 211-220.
- Aldercreutz H. Western diet and Western disease: some hormonal and biochemical mechanisms and associations. Scand. J. Clin. Lab. Investig. 1990; 50(Suppl. 201): 3–23.
- Miller AB. Diet and Cancer. A review. Acta Oncol. 1990; 29(1): 87–95.
- Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Micronutrient synergy — A new tool in effective control of metastasis and other key mechanisms of cancer. Cancer Metastasis Rev. 2010; 29(3): 529–543.
- Rath M, Pauling L. Plasmin-induced proteolysis and the role of apoprotein(a), lysine and synthetic analogs. Orthomol. Med. 1992; 7(1): 17–23.
- Gibellini L, Pinti M, Nasi M, et al. Quercetin and cancer chemoprevention. Evid. Based Complement. Altern. Med. 2011, Article ID 591356
- Kale A, Gawande S, Kotwal S, et al. A combination of green tea extract, specific nutrient mixture and quercetin: An effective intervention treatment for the regression of N-methyl-N-nitrosourea (MNU)-induced mammary tumors in Wistar rats. Oncol Lett. 2010; 1(2): 313-317.
- Cha J, Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int J Oncol. 2013; 42(1): 55-64.

