Scientific basis of micronutrient applications as an effective, safe, and affordable global public health strategy to help control the coronavirus pandemic
Global impact of COVID-19 pandemic
Over 70 million cases of COVID-19 had been confirmed worldwide by mid-December 2020, and the disease had claimed more than 1.6 million lives. According to a joint statement issued on 13 October 2020 by ILO, FAO, IFAD and WHO “the COVID-19 pandemic has led to a dramatic loss of human life worldwide and presents an unprecedented challenge to public health, food systems and the world of work. The economic and social disruption caused by the pandemic is devastating: tens of millions of people are at risk of falling into extreme poverty, while the number of undernourished people, currently estimated at nearly 690 million, could increase to over 800 million by the end the year”.1 In addition, the wide socio-economic impact of this pandemic has threatened the sustainability of all sectors of society all over the globe, impacting mental health, the food supply, medical services, education, and energy, among many other aspects.2
This statement highlights the important, but often overlooked, fact that healthy nutrition supplying a full spectrum of micronutrients is the foundation of our immune system function and provides effective protection against infections, including COVID-19.
Cellular mechanisms of SARS-CoV-2 infection
The virus identified as the cause of COVID-19 pandemic is a type of coronavirus designated as SARS-CoV-2 (see Figure 1). In infected people it causes a serious respiratory illness known as Severe Acute Respiratory Syndrome (SARS) that has fatal consequences.
The entry of the SARS-CoV-2 virus into the cell involves the binding of a viral Spike glycoprotein to its cellular receptor, ACE2 (angiotensin-converting enzyme 2) (see Figure 2).3 ACE2 is present on many cell types throughout the human body, with strong expression in alveolar cells in the lungs, nasal epithelial cells, as well as cells in the heart, blood vessels, and other organs.4,5 Recently, another cellular host receptor for the SARS-CoV-2 virus, known as Neuropilin-1 (NRP-1), has been identified by Daly et al.6 This receptor is abundantly expressed in endothelial and epithelial cells in the respiratory tract and is involved in the SARS-CoV-2 infectivity process.7
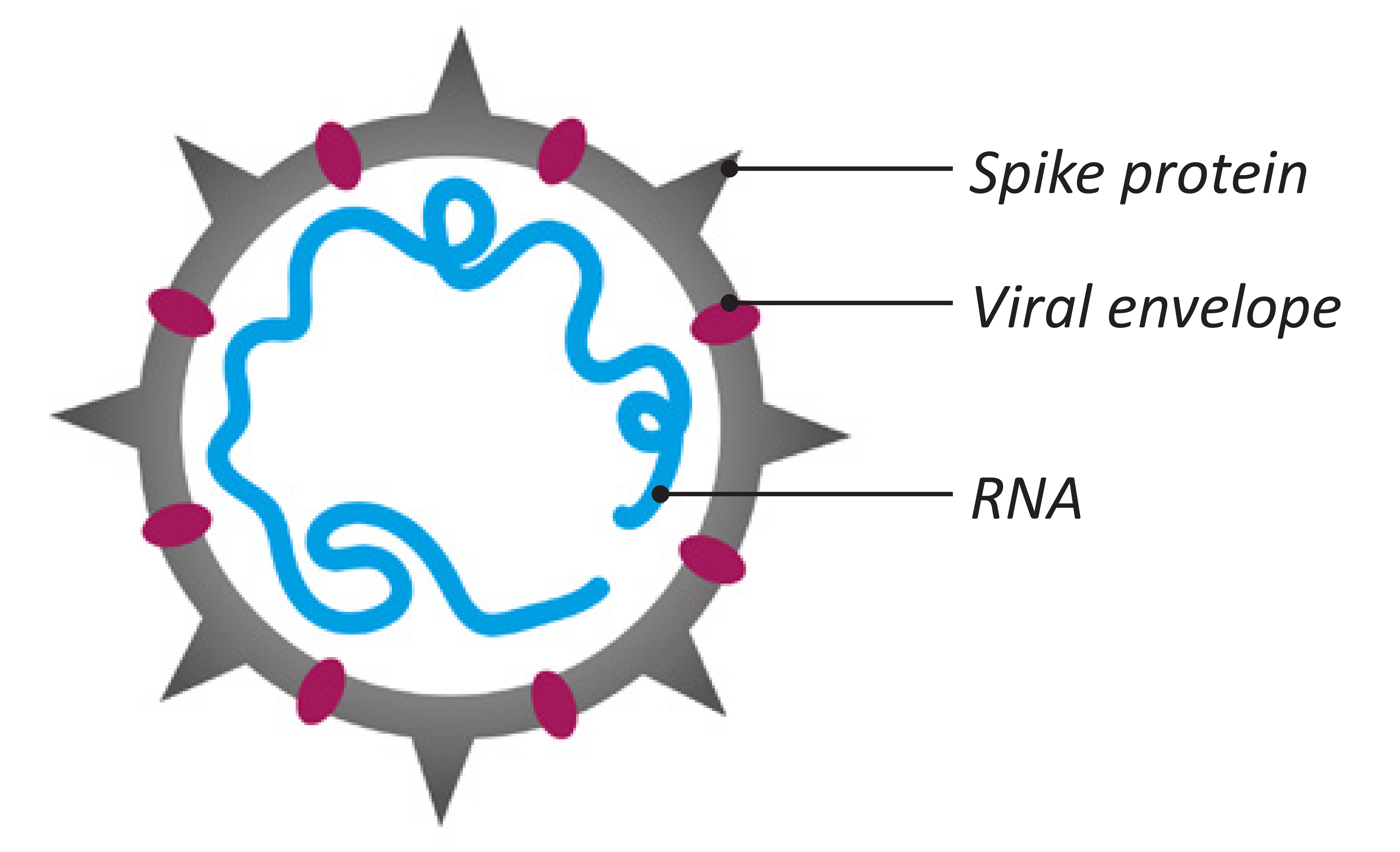
Figure 1: Coronavirus structure
The viral binding to cellular receptors occurs through a specific sequence on the SARS-CoV-2 Spike protein known as the receptor-binding-domain (RBD), which determines viral infectivity and forms the potential target for therapeutic intervention and vaccination (see Figure 3). The RBD located on the S1 subunit of the viral Spike recognizes the ACE2 receptor, which is associated with its conformational changes.8 The RBD stands up and keeps the S1 protein domain in an open conformational state to initiate attachment of the virus to the human ACE2. This conformational change of RBD results in fusion with the host cell membrane through another (the S2) subunit of the Spike protein. The S2 subunit helps keep the virus in conformational changes during the fusion process of the virus after endocytosis.9,10
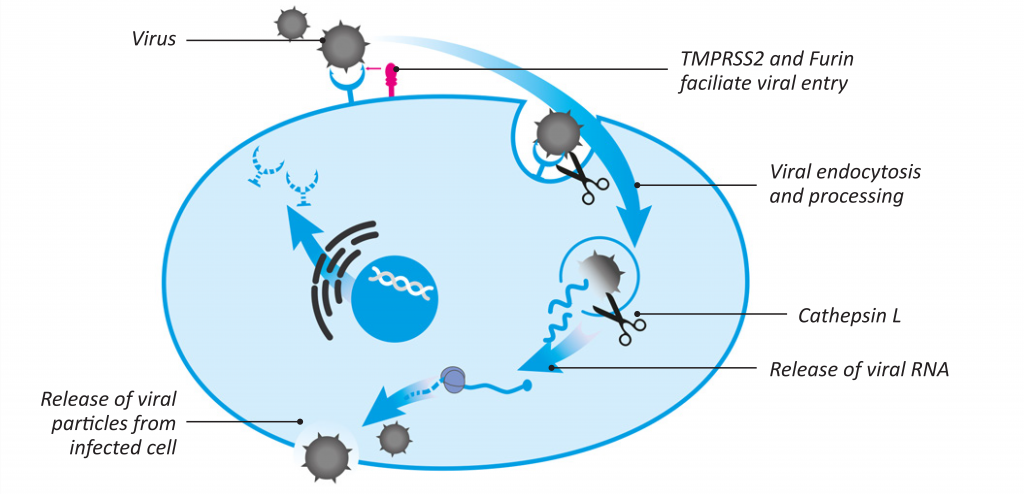
Figure 2: Key stages of SARS-CoV-2 infection of human cells
As presented in Figure 2, both the binding and viral processing inside the cells involve several host proteases, such as the Type II transmembrane serine protease (TMPRSS2), furin, cathepsin L, and others.11,12
Once inside the cell, SARS-CoV-2 uses its RNA-dependent RNA polymerase (RdRp) to translate its genetic material (RNA) and replicate the viral genome.13
Current approaches to COVID-19 pandemic
Despite so much advancement in medicine, science, and technology, we still rely on basic social distancing and isolation methods to limit viral spread. Medical intervention and treatments have brought mixed results, with only some improvements in some countries.
The most recent strategy to end the COVID-19 pandemic relies on vaccines developed using experimental RNA- and DNA-based technology. These vaccines are different from traditional vaccines, which contain weakened or killed viruses. Instead, they contain only a genetic blueprint (RNA or DNA) of the Spike protein of SARS-CoV-2. The DNA-based vaccines use viral DNA packed into another type of virus – generally adenoviruses – with the goal of introducing the viral gene-fragment into the human body to initiate the production of viral protein and trigger an immune response. In RNA-based vaccines the mRNA encoding the viral Spike protein is packaged in lipid nanoparticles in order to enter the cell, where it is translated by host ribosomes into the viral protein. This protein is further processed inside the cell to trigger T-cell immunity and the production of antibodies.
Since the introduction of mRNA vaccines there have been reports of mostly mild transient side effects (fever, headache, etc.). However, some patients experienced a severe allergic reaction immediately after the injection. Facial nerve paralysis and inflammation of the spine have also been reported.14 At the same time, the long-term immune efficacy and side effects of this vaccination are not known. All these add to public skepticism and resistance to accepting the vaccination.
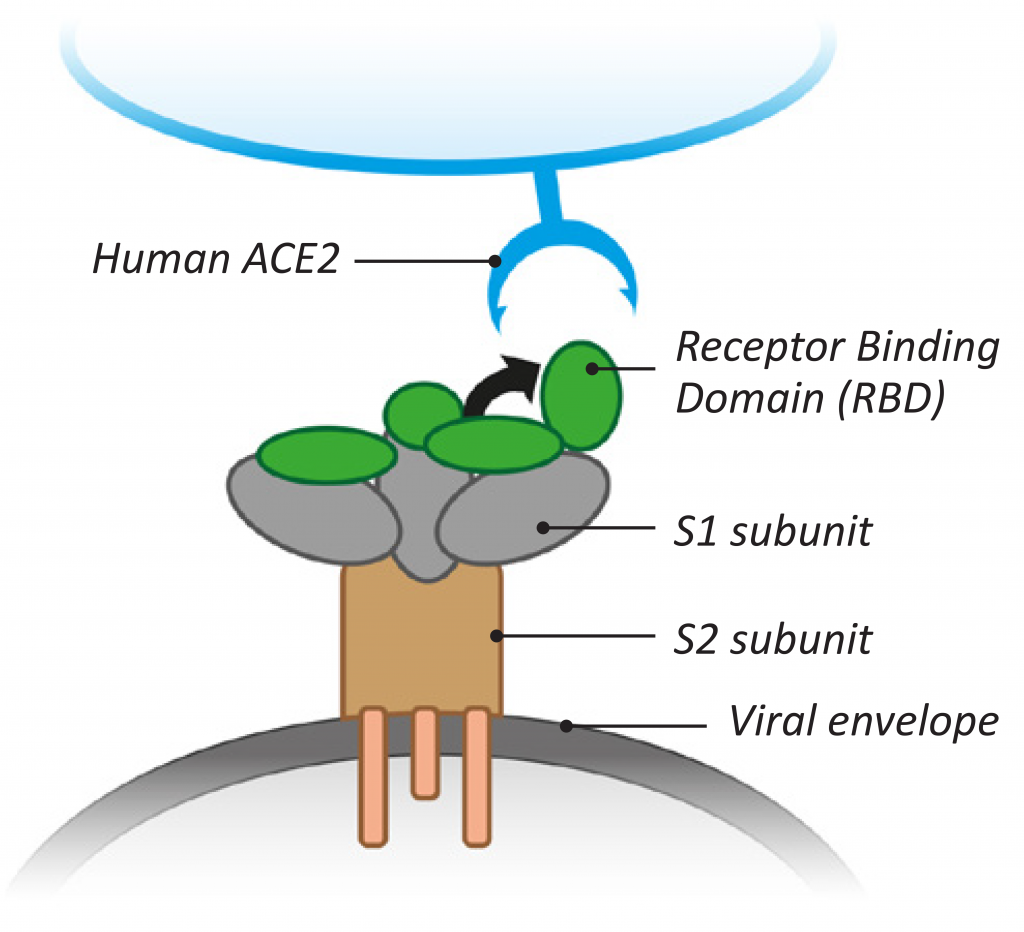
Figure 3: Features of SARS-CoV-2 binding to ACE2 cellular receptors
The need for safe and effective strategies to control and prevent the coronavirus pandemic
Impaired immunity is a major risk factor for infection with and death from coronavirus. Based on multiple reports, the elderly and individuals with comorbidities such as hypertension, diabetes, cancer, and obesity are more severely affected by COVID-19 and suffer more health complications.15-17 All these risk factors are associated with micronutrient deficiencies due to inadequate diets, diabetes and other genetic predispositions, the intake of various pharmaceutical drugs, smoking, environmental pollution, and other external factors. Collectively, they culminate in metabolic impairments and altering the health status of affected individuals, including immunity.
At the beginning of COVID-19 pandemic, the correlation between low vitamin D levels, zinc deficiency, and a risk of infections was established.18 Zinc undernutrition or deficiency was shown to impair cellular mediators of innate immunity such as phagocytosis, natural killer cell activity, and the generation of oxidative burst.19 Its combination with vitamin C showed immune enhancing effects.20
Among various immune protective mechanisms attributed to vitamin D, this nutrient affects the cellular immune system by controlling the overproduction of cytokines triggered by viral infections, including COVID-19. Vitamin D can also decrease tissue damage induced by the ‘cytokine storm’.21 It is interesting that a combination of vitamin C with amino acids, green tea extract, and other micronutrients showed benefits in decreasing cellular receptors for the SARS-CoV-2 virus in lung cells under pro-inflammatory conditions.22,23
It is well established that vitamin C has strong antiviral effects and that it alleviates an inflammation process known to be aggravated in coronavirus infections resulting in the life-threatening ‘cytokine storm’.24,25 Together with vitamin D and zinc, vitamin C interacts synergistically in protecting the integrity of biological barriers guarding against pathogen entry and supporting various immune cell functions.26 Among other nutrients, B vitamins are needed for an optimum cellular response to viral infections and antibody production by white blood cells.27 As such, it has been shown that vitamin B6 deficiency impairs both humoral and cell-mediated immunity by affecting lymphocyte proliferation, differentiation and maturation, as well as cytokine and antibody production.28,29 Elderly people in particular are prone to vitamin B12 and folate deficiency, which negatively affect their immunity status.30,31 Various plant extracts and active plant components have also shown beneficial effects on immune system function and are safe.32 Among these substances, a sulfated polysaccharide from brown algae (fucoidan) shows a variety of immune-modulatory effects, including activation of various immune system cells and enhancing anti-viral responses. It is involved in immune activities such as those of macrophages, NK cells, and cytokines.33,34 Polyphenol and vitamin C-rich tart cherries, lychee fruits, ginger root, and others have similarly shown strong anti-inflammatory and antioxidant effects in human and animal studies.35
Advantages of micronutrients against coronaviruses
Since the onset of the COVID-19 pandemic, there has been an increasing interest in applying safe and effective natural approaches that would support immune system function and have direct antiviral effects on the coronavirus. Micronutrients, especially when applied in specific combinations, can both affect the infectious agent directly and improve immune system functions, thus increasing efficacy in the elimination of viruses and other pathogens. Since their key target is to strengthen body cells in their defense against viral attacks in general, micronutrients are also effective towards mutated coronaviruses. In addition, micronutrients have a large margin of safety and support multiple cellular functions in the body.
Since the onset of COVID-19, various studies have searched for individual natural compounds that could prevent the first step of infection – the binding of the Spike protein on the virus to its natural cellular receptor. Many of these studies applied molecular modelling methods (a “key = viral Spike protein” and “lock = cellular receptor” approach),17 or conducted theoretical evaluations based on the known efficacy of individual compounds in other types of infections.36 A limited number involved experimental tests evaluating which micronutrients interfere with the binding of the COVID Spike protein to its specific receptor on the cell surface.37,38
Clinical applications of micronutrients against coronaviruses
Many studies recommend the consumption of vitamin C to control lower respiratory tract infections. Vitamin C supplementation represents one of the most compelling therapeutic interventions against coronaviruses.39-42
A clinical trial in the USA reported that intravenous (IV) doses of vitamin C decreased sepsis-induced Acute Respiratory Distress Syndrome (ARDS) death rates. ARDS is a life-threatening lung injury that allows fluid to leak into the lungs, impairing breathing and decreasing oxygen supply to the body. The development of ARDS in patients with COVID-19 is a critical complication that leads to mortality.43
A recently published randomized, placebo-controlled clinical intervention study documented that high dose vitamin C can cut the death rate in patients with advanced stages of COVID-19 almost in half.44
This multi-center clinical study coordinated by the University Hospital of Wuhan, the site of the outbreak of the current pandemic, included COVID-19 patients confined to intensive care units due to the severity of the life-threatening stage of their infections. In these severely ill patients, daily doses of 24 grams of vitamin C, given intravenously, were able to cut the death rate (mortality) in about half, compared to those patients receiving only a placebo.
Patients receiving this high-dose vitamin C treatment also had a significantly better oxygenation of their blood, indicating that oxygen can better move to the red blood cells across the lung tissue. That in turn, means that the lung tissue is less inflamed, a fact that was confirmed in this study by much lower levels of inflammation markers (Interleukin-6) in the vitamin C patients.
Particularly significant, however, was the much better chance of surviving COVID-19 for those patients that received vitamin C. Earlier, while an antiviral drug (Remdesivir) was officially approved in COVID-19 patients, a November 2020 statement by the WHO indicated there is currently no evidence that Remdesivir improves survival and other outcomes in COVID-19 patients.45 In contrast, based on the difference in death rates between those patients receiving vitamin C and those who did not, the vitamin C study provided an objective criterium of vitamin C efficacy.
Since the emergence of the COVID-19 pandemic, several clinical trials with vitamins C, D, A, B3, and zinc have also been initiated in different countries, as listed in WHO records.46
The clinical applications of vitamin C document its therapeutic efficacy in COVID-19 patients but without specifically addressing its cellular mechanisms. In general, the benefits of vitamin C in the immune response are based on its potent antioxidant properties, as a cofactor for various enzymes involved in biosynthesis and gene regulation processes, and as an essential factor in collagen synthesis and integrity, thereby optimizing natural biological barriers against infectious agents. Our studies show that vitamin C also inhibits several key mechanisms of coronavirus infections, including down-regulating the viral door- openers (ACE2 receptors) on the surface of human body cells.22 Moreover, we have shown that by combining vitamin C with certain other micronutrients, these effects can be significantly enhanced.23
Designing micronutrient combinations against SARS-CoV-2 towards natural control of the coronavirus pandemic
By combining specific micronutrients that have different cellular functions we can achieve enhanced antiviral efficacy. Such nutrient combinations can simultaneously control a variety of cellular processes associated with infection and are effective at lower doses than when used individually. This principle of ‘nutrient synergy’ has formed the backbone of our scientific research for over two decades, with clinical evidence of its efficacy in other viral and bacterial infections.47-49
In a recent study we evaluated the effects of a composition of plant extracts and active plant components that include curcumin, resveratrol, green tea extract, cruciferous plant extracts, and quercetin, on key cellular mechanisms involved in SARS-CoV-2 infectivity (Figure 4).
A. Micronutrients in decreasing ACE2 and NRP-1 receptors
Decreasing ACE2 and NRP-1 receptors (‘SARS-CoV-2 docking stations’) presents an important therapeutic goal for decreasing viral infectivity.
Our previous studies demonstrated that a specific combination of micronutrients can inhibit the expression of ACE2 receptors on human alveolar epithelial cells by 90%.22,23 In a more recent study, we demonstrated that these micronutrients can also decrease the expression of NR-1 receptors in cells overexpressing ACE2 proteins.38
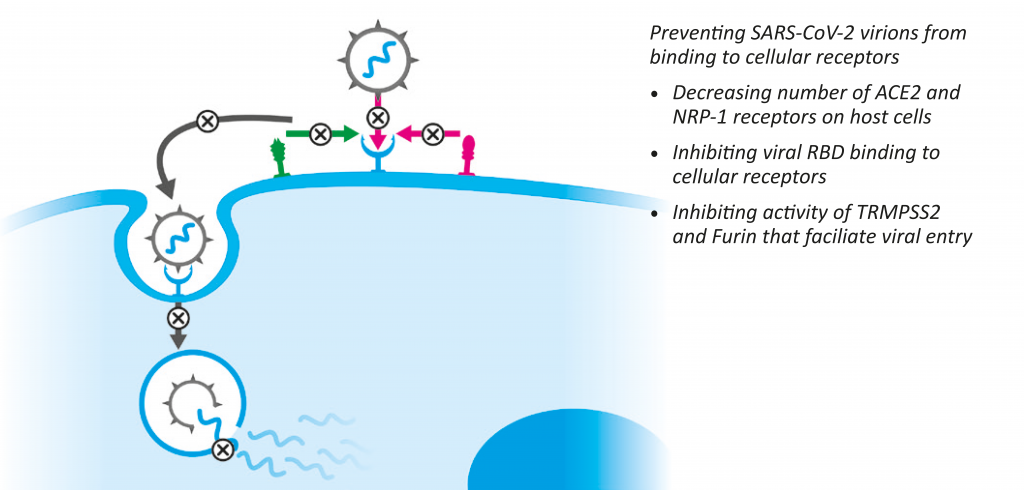
Figure 4: Micronutrients affect key mechanisms involved in SARS-CoV-2 infection of human cells
In addition, we observed that vitamin C (ascorbic acid) used at high (up to 10 mM) concentrations can significantly decrease ACE2 production at the protein and RNA levels, and enhance the efficacy of other natural compounds (i.e. green tea extracts (EGCG), baicalein, curcumin, and others) in decreasing cellular ACE2 expression (Ivanov et a l., submitted). Speth demonstrated that zinc exposure (100 µM) can reduce recombinant human ACE2 activity in rat lungs.
However, the modulating effect of zinc on the SARS-CoV-2/ACE2 interaction seem to be only hypothetical.50 Our study shows that the efficacy of zinc in decreasing ACE2 expression can be potentiated by its combination with vitamin C. As such, zinc aspartate applied at a concentration of 33 µM results in ACE2 inhibition by 22%. When combined with ascorbate, however, this inhibitory effect more than doubles and results in a 62% ACE2 inhibition.23
Interestingly, in September 2020 a clinical prospective randomized study with 4500 participants was initiated at the Mayo Clinics to test the role of zinc versus multivitamin supplementation in supporting immune health in the context of the COVID-19 pandemic. The results are expected in September 2021.51
B. Micronutrients inhibit the RBD binding to cellular ACE2 receptors
This mechanism of the RBD regarding the S-protein binding to host cells provides the framework for the design of inhibitors to prevent entry of the virus, thus curbing further infections in the host.
Our studies show that various natural components, such as vitamins and fatty acids, can directly interfere with viral RBD binding to ACE2 receptors.38 In addition, we could demonstrate that a specific combination of plant-derived compounds can inhibit SARS-CoV-2 pseudo virus from binding to cells expressing ACE2 receptors, when applied both before and after this virus enters the cells.38 These data confirm the high efficacy of natural compounds in the prevention of this key step in the viral infectivity of new cells, as well as their efficacy in already-infected cells.
Micronutrients in inhibiting TMPRSS2, furin, and cathepsin L activities
The endosomal protease cathepsin L, or the cell membrane-associated serine protease TMPRSS2 and furin, have been implicated in facilitating SARS-CoV virus entry into host cells by cleavage of the viral S protein.52 Therefore, interference with the activity of these proteases is required for efficient inhibition of virus infectivity and pathogenesis. In addition to SARSCoV- 2 infection, the potential signal link between the Spike protein, furin, and ACE2 has been implied in the occurrence of adverse cardiovascular events.53
Our studies showed that natural compounds can inhibit the activity of these enzymes when tested directly and in the cells.38
Micronutrients in inhibiting RdRp polymerase
In vitro experiments demonstrate that zinc possesses antiviral activity through inhibition of SARS CoV RNA polymerase. Specifically, Zn²⁺ cations – especially in combination with zinc ionophore pyrithione – were shown to inhibit SARS-coronavirus RNA polymerase (RNA-dependent RNA polymerase, RdRp) activity by decreasing its replication.54
In our study, the defined combination of micronutrients could inhibit RdRp activity by 100%.38
CONCLUSION
These and other findings confirm the significant potential of micronutrients, especially when applied in a specific combination, as a new therapeutic strategy in controlling the COVID-19 pandemic. This direction shows superiority to other currently applied measures by simultaneously affecting key infection mechanisms used by SARS-CoV-2 and other coronaviruses: viral entry, its replication potential, and the expression of cellular ACE2 receptors. In addition, the general safety of natural compounds makes this approach a safe and effective alternative that can be used by the general public. Health practitioners in particular should consider micronutrient deficiencies as a key factor when evaluating patients with COVID-19 conditions. Since screening for these deficiencies can be difficult or impractical, it is prudent to assume nutrient compromising status in all patients and to apply targeted micronutrient supplementation as a general strategy to COVID-19.
References
- Impact of COVID-19 on people’s livehoods, their health and our food systems. Word Health Organization Website.
https://www.who.int/news/item/13-10-2020-impact-of-covid-19-on-people‘s-livelihoods-their-health-and-our-food-
systems Published October 13, 2020. Accessed January 13, 2021. - Nicola M, Alsafi Z, Sohrabi C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): A
review. Int J Surg. 2020;78:185-193. doi:10.1016/j.ijsu.2020.04.018 - Tai W, He L, Zhang X, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613-620. doi:10.1038/s41423-020-0400-4
- Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681-687. doi:10.1038/s41591-020-0868-6
- Mahmoud IS, Jarrar YB, Alshaer W, Ismail S. SARS-CoV-2 entry in host cells-multiple targets for treatment and prevention. Biochimie. 2020;175:93-98. doi:10.1016/j.biochi.2020.05.012
- Daly JL, Simonetti B, Klein K, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370(6518):861-865. doi:10.1126/science.abd3072
- Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856-860. doi:10.1126/science.abd2985
- Lin L, Ting S, Yufei H, Wendong L, Yubo F, Jing Z. Epitope-based peptide vaccines predicted against novel coronavirus disease caused by SARS-CoV-2. Virus Res. 2020;288:198082. doi:10.1016/j.virusres.2020.198082
- Laha S, Chakraborty J, Das S, Manna SK, Biswas S, Chatterjee R. Characterizations of SARS-CoV-2 mutational profile, spike protein stability and viral transmission. Infect Genet Evol. 2020;85:104445. doi:10.1016/j.meegid.2020.104445
- Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(21):11727-11734. doi:10.1073/ pnas.2003138117
- Simmons G, Zmora P, Gierer S, Heurich A, Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antiviral Res. 2013;100(3):605-614. doi:10.1016/j.antiviral.2013.09.028
- Bosch BJ, van der Zee R, de Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein:
structural and functional characterization of the fusion core complex. J Virol. 2003;77(16):8801-8811. doi:10.1128/jvi.77.16.8801-8811.2003 - Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1-23. doi:10.1007/978-1-4939-2438-7_1
- Allen A, Kaiser LS. NIH ‘Very concerned’ about serious side effect in Coronavirus vaccine trial. Scientific American. September 15, 2020. https://www.scientificamerican.com/article/nih-very-concerned-about-serious-side-effect-in-coronavirus-vaccine-trial/ Accessed January 13, 2021.
- Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi:10.1016/j.jcv.2020.104371
- Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health. 2020;13(12):1833-1839. doi:10.1016/j.jiph.2020.07.014
- Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766-788. doi:10.1016/j.apsb.2020.02.008
- Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open. 2020;3(9):e2019722. Published 2020 Sep 1.
doi:10.1001/jamanetworkopen.2020.19722 - Wessels I, Rolles B, Rink L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front Immunol. 2020;11:1712. Published 2020 Jul 10. doi:10.3389/fimmu.2020.01712
- Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85-94. doi:10.1159/000090495
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30;:]. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-
6736(20)30183-5 - Ivanov V, Ivanova S, Niedzwiecki A, Rath M, Effective and safe global public health strategy to fight the COVID19 pandemic: Specific micronutrient composition inhibits Coronavirus cell-entry receptor (ACE2) expression, J Cellular Medicine and Natural Health. 2020.
- Ivanov V, Goc A, Ivanova S, Niedzwiecki A, M. Rath R, Inhibition of coronavirus receptor ACE2 expression by
ascorbic acid alone and in combinations with other natural compounds. 2020. Submitted. - Boretti A, Banik BK. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 2020;12:100190. doi:10.1016/j.phanu.2020.100190
- Ang A, Pullar JM, Currie MJ, Vissers MCM. Vitamin C and immune cell function in inflammation and cancer.
Biochem Soc Trans. 2018;46(5):1147-1159. doi:10.1042/BST20180169 - Name JJ, Souza ACR, Vasconcelos AR, Prado PS, Pereira CPM. Zinc, Vitamin D and Vitamin C: Perspectives for COVID-19 With a Focus on Physical Tissue Barrier Integrity. Front Nutr. 2020;7:606398. Published 2020 Dec 7.
doi:10.3389/fnut.2020.606398 - Shane B. Folic acid, vitamin B-12, and vitamin B-6. In: Stipanuk M, ed. Biochemical and Physiological Aspects of
Human Nutrition. 2nd ed. Philadelphia: Saunders Elsevier; 2006:693-732. - Chandra RK, Sudhakaran L. Regulation of immune responses by vitamin B6. Ann N Y Acad Sci. 1990;585:404-
423. doi:10.1111/j.1749-6632.1990.tb28073.x - Rall LC, Meydani SN. Vitamin B6 and immune competence. Nutr Rev. 1993;51(8):217-225. doi:10.1111/j.1753-4887.1993.tb03109.x
- Gross RL, Reid JV, Newberne PM, Burgess B, Marston R, Hift W. Depressed cell-mediated immunity in megaloblastic anemia due to folic acid deficiency. Am J Clin Nutr. 1975;28(3):225-232. doi:10.1093/ajcn/28.3.225
- Erkurt MA, Aydogdu I, Dikilitaş M, et al. Effects of cyanocobalamin on immunity in patients with pernicious anemia. Med Princ Pract. 2008;17(2):131-135. doi:10.1159/000112967
- Sumera W, Goc A, Niedzwiecki A, Rath M, The micronutrient combination with immune-enhancing effects. J Cellular Medicine and Natural Health. 2020.
- Maruyama H, Tamauchi H, Iizuka M, Nakano T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med. 2006;72(15):1415-1417. doi:10.1055/s-2006-951703
- Li B, Lu F, Wei X, Zhao R. Fucoidan: structure and bioactivity. Molecules. 2008;13(8):1671-1695. Published 2008 Aug 12. doi:10.3390/molecules13081671
- Kelley DS, Adkins Y, Laugero KD. A Review of the Health Benefits of Cherries. Nutrients. 2018;10(3):368. Published
2018 Mar 17. doi:10.3390/nu10030368 - Quiles JL, Rivas-García L, Varela-López A, Llopis J, Battino M, Sánchez-González C. Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19?. Environ Res. 2020;191:110053. doi:10.1016/j.envres.2020.110053
- Goc A, Sumera W, Ivanov V, Niedzwiecki A, Rath M. Micronutrient combination inhibits two keys steps of coronavirus (SARS_CoV-2) infection: vital binding to ACE2 receptor and its cellular receptor, J Cellular Medicine and Natural Health. 2020.
- Goc A, Ivanov V, Ivanova S, Chatterjee M, Rath M, Niedzwiecki A, Simultaneous inhibition of key mechanisms of SARS- CoV-2 infection by a specific combination of plant-derived compounds. 2020 (submitted).
- Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020;8(5):433-434. doi:10.1016/S2213-2600(20)30127-2
- Zhang J, Xie B, Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis.
Brain Behav Immun. 2020;87:59-73. doi:10.1016/j.bbi.2020.04.046 - Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front Immunol. 2020;11:1451. Published 2020 Jun 19. doi:10.3389/fimmu.2020.01451
- Liu F, Zhu Y, Zhang J, Li Y, Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10(7):e039519. Published 2020 Jul 8. doi:10.1136/bmjopen-2020-039519
- Fowler AA 3rd, Truwit JD, Hite RD, et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRISALI Randomized Clinical Trial [published correction appears in JAMA. 2020 Jan 28;323(4):379]. JAMA. 2019;322(13):1261-1270. doi:10.1001/jama.2019.11825
- Zhang J, Rao X, Li Y, et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021;11(1):5. Published 2021 Jan 9. doi:10.1186/s13613-020-00792-3
- Who recommends against the use of remdesivir in covid-19 patients. World Health Organization. https://www.who.int/news-room/feature-stories/detail/who-recommends-against-the-use-of-remdesivir-in-covid-
19-patients. Published January, 2021. - Covid-19 studies from the world health organization database. https://clinicaltrials.gov/ct2/who_table
- Goc A, Gehring G, Baltin H, Niedzwiecki A, Rath M. Specific composition of polyphenolic compounds with fatty acids as an approach in helping to reduce spirochete burden in Lyme disease: in vivo and human observational study. Ther Adv Chronic Dis. 2020;11:2040622320922005. Published 2020 May 24. doi:10.1177/2040622320922005
- Jariwalla RJ, Niedzwiecki A, Rath M. The essentiality of nutritional supplementation in HIV infection and AIDS: Review of clinical studies and results from a community health micronutrient program. Bioactive Foods in Promoting Health. 323-342. 10.1016/B978-0-12-374628-3.00022-0.
- Turchenko LV, Voloshchuk EO, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Clinical improvement of active tuberculosis patients with complex treatment and nutritional supplementation. The Open Natural Products Journal. 2008;1: 20-26.
- Speth R, Carrera E, Jean-Baptiste M, Joachim A, Linares A. Concentration-dependent effects of zinc on angiotensin-converting enzyme-2 activity (1067.4). FASEB J. 2014; 28(Suppl 1): 1067.42014.
- Zinc versus multivitamin micronutrient supplementation in the setting of COVID-19 (ZNCOVID-19). ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04551339 Published September 16, 2020. Updated January 5, 2021.
Accessed January 13, 2021. - Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is
Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-280.e8. doi:10.1016/j.cell.2020.02.052 - Ming Y, Qiang L. Involvement of Spike Protein, Furin, and ACE2 in SARS-CoV-2-Related Cardiovascular Complications [published online ahead of print, 2020 Jul 11]. SN Compr Clin Med. 2020;1-6. doi:10.1007/s42399-020-00400-2
- te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176. Published 2010 Nov 4. doi:10.1371/journal.ppat.1001176
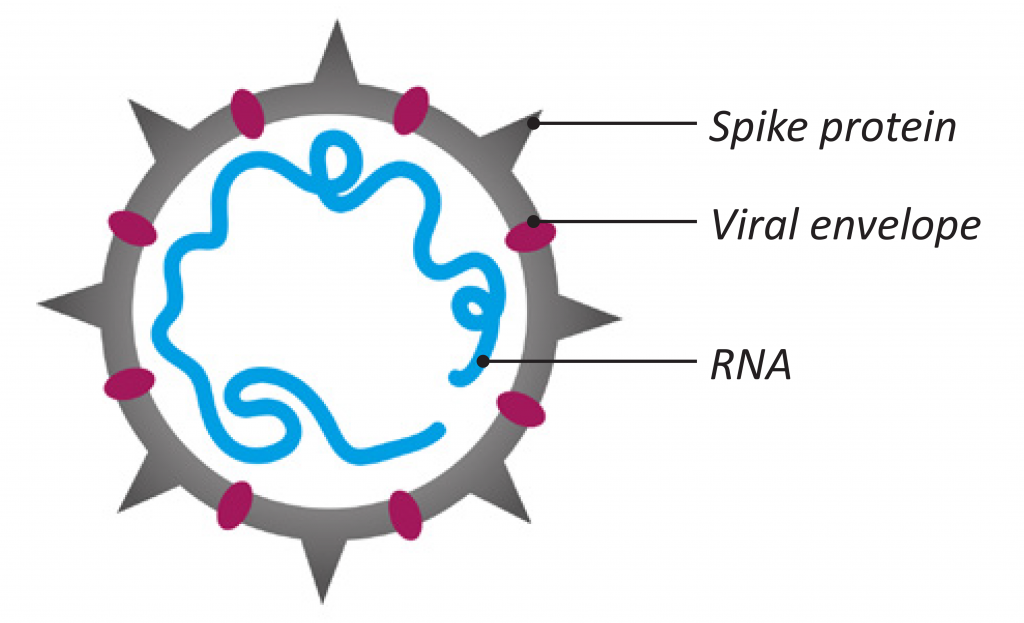
Figure 1: Coronavirus structure

Figure 2: Key stages of SARS-CoV-2 infection of human cells

Figure 3: Features of SARS-CoV-2 binding to ACE2 cellular receptors

Figure 4: Micronutrients affect key mechanisms involved in SARS-CoV-2 infection of human cells
References
- Impact of COVID-19 on people’s livehoods, their health and our food systems. Word Health Organization Website.
https://www.who.int/news/item/13-10-2020-impact-of-covid-19-on-people‘s-livelihoods-their-health-and-our-food-
systems Published October 13, 2020. Accessed January 13, 2021. - Nicola M, Alsafi Z, Sohrabi C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): A
review. Int J Surg. 2020;78:185-193. doi:10.1016/j.ijsu.2020.04.018 - Tai W, He L, Zhang X, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613-620. doi:10.1038/s41423-020-0400-4
- Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681-687. doi:10.1038/s41591-020-0868-6
- Mahmoud IS, Jarrar YB, Alshaer W, Ismail S. SARS-CoV-2 entry in host cells-multiple targets for treatment and prevention. Biochimie. 2020;175:93-98. doi:10.1016/j.biochi.2020.05.012
- Daly JL, Simonetti B, Klein K, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370(6518):861-865. doi:10.1126/science.abd3072
- Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856-860. doi:10.1126/science.abd2985
- Lin L, Ting S, Yufei H, Wendong L, Yubo F, Jing Z. Epitope-based peptide vaccines predicted against novel coronavirus disease caused by SARS-CoV-2. Virus Res. 2020;288:198082. doi:10.1016/j.virusres.2020.198082
- Laha S, Chakraborty J, Das S, Manna SK, Biswas S, Chatterjee R. Characterizations of SARS-CoV-2 mutational profile, spike protein stability and viral transmission. Infect Genet Evol. 2020;85:104445. doi:10.1016/j.meegid.2020.104445
- Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(21):11727-11734. doi:10.1073/ pnas.2003138117
- Simmons G, Zmora P, Gierer S, Heurich A, Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antiviral Res. 2013;100(3):605-614. doi:10.1016/j.antiviral.2013.09.028
- Bosch BJ, van der Zee R, de Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein:
structural and functional characterization of the fusion core complex. J Virol. 2003;77(16):8801-8811. doi:10.1128/jvi.77.16.8801-8811.2003 - Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1-23. doi:10.1007/978-1-4939-2438-7_1
- Allen A, Kaiser LS. NIH ‘Very concerned’ about serious side effect in Coronavirus vaccine trial. Scientific American. September 15, 2020. https://www.scientificamerican.com/article/nih-very-concerned-about-serious-side-effect-in-coronavirus-vaccine-trial/ Accessed January 13, 2021.
- Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi:10.1016/j.jcv.2020.104371
- Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health. 2020;13(12):1833-1839. doi:10.1016/j.jiph.2020.07.014
- Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766-788. doi:10.1016/j.apsb.2020.02.008
- Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open. 2020;3(9):e2019722. Published 2020 Sep 1.
doi:10.1001/jamanetworkopen.2020.19722 - Wessels I, Rolles B, Rink L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front Immunol. 2020;11:1712. Published 2020 Jul 10. doi:10.3389/fimmu.2020.01712
- Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85-94. doi:10.1159/000090495
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30;:]. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-
6736(20)30183-5 - Ivanov V, Ivanova S, Niedzwiecki A, Rath M, Effective and safe global public health strategy to fight the COVID19 pandemic: Specific micronutrient composition inhibits Coronavirus cell-entry receptor (ACE2) expression, J Cellular Medicine and Natural Health. 2020.
- Ivanov V, Goc A, Ivanova S, Niedzwiecki A, M. Rath R, Inhibition of coronavirus receptor ACE2 expression by
ascorbic acid alone and in combinations with other natural compounds. 2020. Submitted. - Boretti A, Banik BK. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 2020;12:100190. doi:10.1016/j.phanu.2020.100190
- Ang A, Pullar JM, Currie MJ, Vissers MCM. Vitamin C and immune cell function in inflammation and cancer.
Biochem Soc Trans. 2018;46(5):1147-1159. doi:10.1042/BST20180169 - Name JJ, Souza ACR, Vasconcelos AR, Prado PS, Pereira CPM. Zinc, Vitamin D and Vitamin C: Perspectives for COVID-19 With a Focus on Physical Tissue Barrier Integrity. Front Nutr. 2020;7:606398. Published 2020 Dec 7.
doi:10.3389/fnut.2020.606398 - Shane B. Folic acid, vitamin B-12, and vitamin B-6. In: Stipanuk M, ed. Biochemical and Physiological Aspects of
Human Nutrition. 2nd ed. Philadelphia: Saunders Elsevier; 2006:693-732. - Chandra RK, Sudhakaran L. Regulation of immune responses by vitamin B6. Ann N Y Acad Sci. 1990;585:404-
423. doi:10.1111/j.1749-6632.1990.tb28073.x - Rall LC, Meydani SN. Vitamin B6 and immune competence. Nutr Rev. 1993;51(8):217-225. doi:10.1111/j.1753-4887.1993.tb03109.x
- Gross RL, Reid JV, Newberne PM, Burgess B, Marston R, Hift W. Depressed cell-mediated immunity in megaloblastic anemia due to folic acid deficiency. Am J Clin Nutr. 1975;28(3):225-232. doi:10.1093/ajcn/28.3.225
- Erkurt MA, Aydogdu I, Dikilitaş M, et al. Effects of cyanocobalamin on immunity in patients with pernicious anemia. Med Princ Pract. 2008;17(2):131-135. doi:10.1159/000112967
- Sumera W, Goc A, Niedzwiecki A, Rath M, The micronutrient combination with immune-enhancing effects. J Cellular Medicine and Natural Health. 2020.
- Maruyama H, Tamauchi H, Iizuka M, Nakano T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med. 2006;72(15):1415-1417. doi:10.1055/s-2006-951703
- Li B, Lu F, Wei X, Zhao R. Fucoidan: structure and bioactivity. Molecules. 2008;13(8):1671-1695. Published 2008 Aug 12. doi:10.3390/molecules13081671
- Kelley DS, Adkins Y, Laugero KD. A Review of the Health Benefits of Cherries. Nutrients. 2018;10(3):368. Published
2018 Mar 17. doi:10.3390/nu10030368 - Quiles JL, Rivas-García L, Varela-López A, Llopis J, Battino M, Sánchez-González C. Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19?. Environ Res. 2020;191:110053. doi:10.1016/j.envres.2020.110053
- Goc A, Sumera W, Ivanov V, Niedzwiecki A, Rath M. Micronutrient combination inhibits two keys steps of coronavirus (SARS_CoV-2) infection: vital binding to ACE2 receptor and its cellular receptor, J Cellular Medicine and Natural Health. 2020.
- Goc A, Ivanov V, Ivanova S, Chatterjee M, Rath M, Niedzwiecki A, Simultaneous inhibition of key mechanisms of SARS- CoV-2 infection by a specific combination of plant-derived compounds. 2020 (submitted).
- Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020;8(5):433-434. doi:10.1016/S2213-2600(20)30127-2
- Zhang J, Xie B, Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis.
Brain Behav Immun. 2020;87:59-73. doi:10.1016/j.bbi.2020.04.046 - Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front Immunol. 2020;11:1451. Published 2020 Jun 19. doi:10.3389/fimmu.2020.01451
- Liu F, Zhu Y, Zhang J, Li Y, Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10(7):e039519. Published 2020 Jul 8. doi:10.1136/bmjopen-2020-039519
- Fowler AA 3rd, Truwit JD, Hite RD, et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRISALI Randomized Clinical Trial [published correction appears in JAMA. 2020 Jan 28;323(4):379]. JAMA. 2019;322(13):1261-1270. doi:10.1001/jama.2019.11825
- Zhang J, Rao X, Li Y, et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021;11(1):5. Published 2021 Jan 9. doi:10.1186/s13613-020-00792-3
- Who recommends against the use of remdesivir in covid-19 patients. World Health Organization. https://www.who.int/news-room/feature-stories/detail/who-recommends-against-the-use-of-remdesivir-in-covid-
19-patients. Published January, 2021. - Covid-19 studies from the world health organization database. https://clinicaltrials.gov/ct2/who_table
- Goc A, Gehring G, Baltin H, Niedzwiecki A, Rath M. Specific composition of polyphenolic compounds with fatty acids as an approach in helping to reduce spirochete burden in Lyme disease: in vivo and human observational study. Ther Adv Chronic Dis. 2020;11:2040622320922005. Published 2020 May 24. doi:10.1177/2040622320922005
- Jariwalla RJ, Niedzwiecki A, Rath M. The essentiality of nutritional supplementation in HIV infection and AIDS: Review of clinical studies and results from a community health micronutrient program. Bioactive Foods in Promoting Health. 323-342. 10.1016/B978-0-12-374628-3.00022-0.
- Turchenko LV, Voloshchuk EO, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Clinical improvement of active tuberculosis patients with complex treatment and nutritional supplementation. The Open Natural Products Journal. 2008;1: 20-26.
- Speth R, Carrera E, Jean-Baptiste M, Joachim A, Linares A. Concentration-dependent effects of zinc on angiotensin-converting enzyme-2 activity (1067.4). FASEB J. 2014; 28(Suppl 1): 1067.42014.
- Zinc versus multivitamin micronutrient supplementation in the setting of COVID-19 (ZNCOVID-19). ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04551339 Published September 16, 2020. Updated January 5, 2021.
Accessed January 13, 2021. - Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is
Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-280.e8. doi:10.1016/j.cell.2020.02.052 - Ming Y, Qiang L. Involvement of Spike Protein, Furin, and ACE2 in SARS-CoV-2-Related Cardiovascular Complications [published online ahead of print, 2020 Jul 11]. SN Compr Clin Med. 2020;1-6. doi:10.1007/s42399-020-00400-2
- te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176. Published 2010 Nov 4. doi:10.1371/journal.ppat.1001176

