Vitamin D enhances anticancer effects of EGCG and a specific micronutrient combination in breast cancer cells
Introduction
Breast cancer is the most common cancer in women worldwide with over 508,000 deaths due to breast cancer in 2011.1
Current breast cancer therapies include localized surgery and radiation, as well as systemic treatments such as chemotherapy and hormone therapy.2 Although newer pharmaceutical options, such as targeted therapy and immunotherapy are being developed, the effective control of breast cancer is still out of reach.3
The use of natural components in cancer therapy has historically been a part of folk medicine, but recent research advancements in biologically active natural ingredients have established a solid scientific base for developing non-toxic and effective cancer approaches.4,5 Studies at the Dr Rath Research Institute documented that a combination of natural components, including vitamin C, polyphenols from green tea, the essential amino acids lysine, proline and arginine, N-acetyl cysteine and others, can affect key mechanisms involved in cancer. Several in vivo and in vitro studies confirmed the efficacy of this nutrient composition in inhibiting cancer cell growth, tissue invasion and metastasis, inducing natural cell death (apoptosis), and curtailing neo-vascularization of tumors in over 55 human cancer cell lines.6
An increasing number of studies indicate the anticancer potential of vitamin D in triggering cancer cell cycle arrest, stimulation of apoptosis and inhibition of invasion, metastasis and angiogenesis.7,8 It has been shown that vitamin D can interact with estrogen receptors and have positive effects in controlling estrogen-dependent breast cancer,9,10 however, specific cellular mechanisms involved are still not sufficiently understood. Also, epidemiological findings show an inverse association of circulating vitamin D levels with breast cancer.11
It is unclear how vitamin D affects the metabolic efficacy of other nutrients and how the presence of other nutrients and metabolites modulates vitamin D efficacy in various cells, particularly cancer cells. This is especially important since cancer patients often use multiple nutrient supplementation in their therapy regimen, the interaction of which is largely unknown.
In our study we investigated the effects of vitamin D used alone and in combination with other nutrients on breast cancer cell growth in vitro. The tests were conducted with MCF-7 human breast cancer cell line which represents estrogen-dependent breast cancer type, allowing vitamin D to interact with estrogen receptors as has been suggested earlier.
Results and Discussion
As presented in Figure 1, the supplementation of cultured breast cancer cells with vitamin D inhibited cancer cell growth in a dose-dependent manner, reaching 32% inhibition at 500 nM, which represents a relatively high value if extrapolated to human blood vitamin D levels. The current recommendation for healthy blood levels of vitamin D is in the range of 100 nM-200 nM. Supplementation with high doses of vitamin D can result in blood vitamin D levels of 375 nM and up to 1.2 μM, which can bring some adverse but reversible health effects.12
Therefore, it was interesting to find out whether vitamin D applied at lower doses but in combination with other micronutrients could enhance the inhibition of breast cancer growth through either synergistic or additive interactions with other nutrients. To this end we tested the effects of epigallocatechin gallate (EGCG)—a component of green tea—alone and in combination with other micronutrients as a nutrient mixture (NM, see Table 1 for composition) both with and without Vitamin D respectively, on the growth of breast cancer cells. The effects of vitamin D in relation to the anticancer effects of these compounds were not known. In our experiments we used a low dose of vitamin D (20 nM) which when applied alone resulted in 17% inhibition of breast cancer cell growth (Figure 1).
The results presented in Figure 2 show that the inhibitory effects of EGCG and NM on cancer cell growth were significantly enhanced by vitamin D. As such, NM used at 33 μg/ml without vitamin D inhibited breast cancer cell growth by 63% and at a concentration of 100 μg/ml by 85%. In the presence of 20 nM vitamin D the rate of breast cancer cell growth further decreased, achieving 76% and 94% inhibition at NM doses of 33 μg/ml and 100 μg/ml, respectively. A 50 μM concentration of EGCG—reportedly one of the most biologically effective polyphenolic compounds of green tea—inhibited breast cancer cell growth by 27%. Combining it with vitamin D further enhanced this inhibitory effect, bringing it to 62%. This means that the inhibitory effects of vitamin D alone on breast cancer cell growth (17%) can be significantly enhanced by combining it with EGCG (62% inhibition) and even more by combining it with NM (up to 94% inhibition). This data also points to potential synergistic effects of a vitamin D/EGCG combination. The effective 62% inhibition achieved with EGCG and Vitamin D used together exceeded the expected sum of the effects of individual components (calculated expectation of 39% inhibition).
The results of our study clearly demonstrate that the anticancer effects of vitamin D could be significantly enhanced by its cooperative interaction with other nutrients with known anticancer activity.
These important findings call for an increased research effort to investigate combinations of biologically active and/ or essential natural nutrients in the prevention and treatment of breast cancer.
Table 1 – Composition of Nutrient Mixture
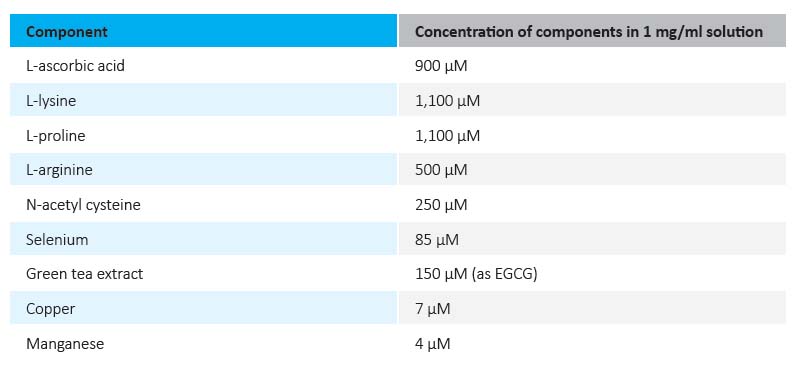
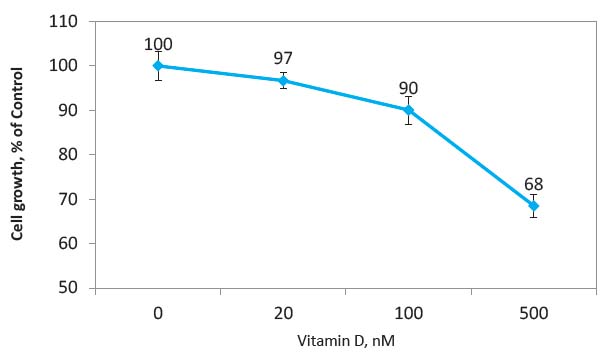
Figure 1 Effects of vitamin D on growth rate of human breast cancer cells (MCF-7), expressed as a percentage of unsupplemented control values ±SD.
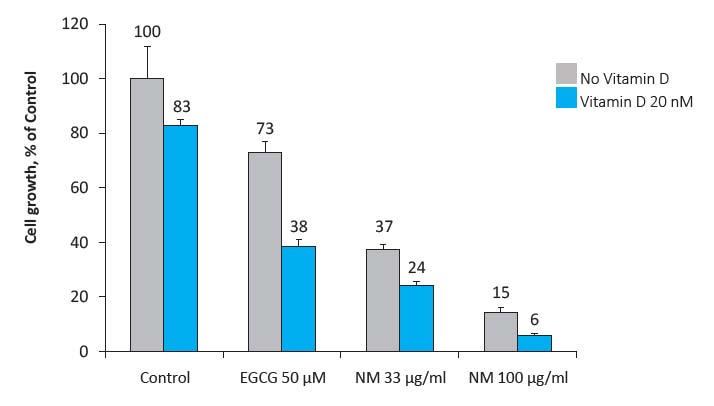
Figure 2 Effects of a combination of vitamin D with EGCG and a specific nutrient mixture (NM) on MCF-7 human breast cancer cells growth. The results are presented as a percentage of control ±SD.
Experimental procedures
Human breast cancer cells (line MCF-7) and cell culture medium components were supplied by ATCC. Nutrient mixture of L-ascorbic acid, L-lysine, L-proline, L-arginine, N-acetyl cysteine, selenium, copper, and green tea extract (GTE) was supplied by Dr Rath Inc. Vitamin D3 (Calcitriol 1,25(OH)2D3), epigallocatechin gallate (EGCG), [H3] thymidine and other reagents were from Sigma-Aldrich.
MCF-7 cells were seeded for experiments in 48 well plastic plates at density 1,000 cells per well in cell growth medium (DMEM supplemented with 2% fetal bovine serum). After attachment cells were incubated for 48 hours at 37oC 5% CO2 with indicated concentrations of tested compounds in cell growth medium supplemented with DNA precursor, [H3] thymidine. Cell growth rate was determined as an amount of [H3] associated cellular DNA, as described previously.13 Results are expressed as a percentage of unsupplemented control values (averages from triplicate samples +/- SD).
References
1. Breast cancer: prevention and control. World Health Organization. https://www.who.int/cancer/detection/breastcancer/en/index1.html Accessed May 5, 2019.
2. Treating Breast Cancer. American Cancer Society. https://www.cancer.org/cancer/breast-cancer/treatment.html Accessed May 5, 2019.
3. Naito Y, Urasaki T. Precision medicine in breast cancer. Chin Clin Oncol. 2018;7(3):29. doi: 10.21037/ cco.2018.06.04.
4. Block G, Patterson B, Sugar A. Fruit, vegetables and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29.
5. Kapinova A, Kubatka P, Golubnitschaja O, et al. Dietary phytochemicals in breast cancer research: anticancer effects and potential utility for effective chemoprevention. Environ Health Prev Med. 2018;23(1):36. https://doi.org/10.1186/s12199-018-0724-1
6. Niedzwiecki A, Rath M. Clinical Nutrients in Cancer Therapy: A Scientific Review and Perspective. Santa Clara, CA: Dr. Rath Research Institute; 2005.
7. Zhang X, Harbeck N, Jeschke U, Doisneau-Sixou S. Influence of vitamin D signaling on hormone receptor status and HER2 expression in breast cancer. J Cancer Res Clin Oncol. 2017;143(7): 1107-1122.
8. Krishnan AV, Swami S, Feldman D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids. 2012;77(11):1107-12. doi: 10.1016/j. steroids.2012.06.005
9. Wu G, Fan RS, Li W, Ko TC, Brattain MG. Modulation of cell cycle control by vitamin D3 and its analogue, EB1089, in human breast cancer cells. Oncogene. 1997;15:1555–1563.
10. Flanagan L, Packman K, Juba B, O’Neill S, Tenniswood M, Welsh J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J Steroid Biochem Mol Biol. 2003;84(2–3):181–192. https://doi.org/10.1016/S0960-0760(03)00028-1
11. Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. Plasma vitamin D levels, menopause, and risk of breast cancer: dose-response metaanalysis of prospective studies. Medicine (Baltimore). 2013;92:123-31. doi: 10.1097/ MD.0b013e3182943bc2
12. Dudenkov DV, Yawn BP, Oberhelman SS, et al. Changing incidence of serum 25-hydroxyvitamin D values above 50 ng/mL: A 10-year population-based study. Mayo Clin Proc. 2015;90(5):577-86. doi: 10.1016/j.mayocp.2015.02.012.
13. Ivanov VO, Ivanova SV, Niedzwiecki A. Ascorbate affects proliferation of guinea-pig vascular smooth muscle cells by direct and extracellular matrix-mediated effects. J Mol Cell Cardiol. 1997; 29(12):3293-303.
Table 1 – Composition of Nutrient Mixture


Figure 1 Effects of vitamin D on growth rate of human breast cancer cells (MCF-7), expressed as a percentage of unsupplemented control values ±SD.

Figure 2 Effects of a combination of vitamin D with EGCG and a specific nutrient mixture (NM) on MCF-7 human breast cancer cells growth. The results are presented as a percentage of control ±SD.
1. Breast cancer: prevention and control. World Health Organization. https://www.who.int/cancer/detection/breastcancer/en/index1.html Accessed May 5, 2019.
2. Treating Breast Cancer. American Cancer Society. https://www.cancer.org/cancer/breast-cancer/treatment.html Accessed May 5, 2019.
3. Naito Y, Urasaki T. Precision medicine in breast cancer. Chin Clin Oncol. 2018;7(3):29. doi: 10.21037/ cco.2018.06.04.
4. Block G, Patterson B, Sugar A. Fruit, vegetables and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29.
5. Kapinova A, Kubatka P, Golubnitschaja O, et al. Dietary phytochemicals in breast cancer research: anticancer effects and potential utility for effective chemoprevention. Environ Health Prev Med. 2018;23(1):36. https://doi.org/10.1186/s12199-018-0724-1
6. Niedzwiecki A, Rath M. Clinical Nutrients in Cancer Therapy: A Scientific Review and Perspective. Santa Clara, CA: Dr. Rath Research Institute; 2005.
7. Zhang X, Harbeck N, Jeschke U, Doisneau-Sixou S. Influence of vitamin D signaling on hormone receptor status and HER2 expression in breast cancer. J Cancer Res Clin Oncol. 2017;143(7): 1107-1122.
8. Krishnan AV, Swami S, Feldman D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids. 2012;77(11):1107-12. doi: 10.1016/j. steroids.2012.06.005
9. Wu G, Fan RS, Li W, Ko TC, Brattain MG. Modulation of cell cycle control by vitamin D3 and its analogue, EB1089, in human breast cancer cells. Oncogene. 1997;15:1555–1563.
10. Flanagan L, Packman K, Juba B, O’Neill S, Tenniswood M, Welsh J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J Steroid Biochem Mol Biol. 2003;84(2–3):181–192. https://doi.org/10.1016/S0960-0760(03)00028-1
11. Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. Plasma vitamin D levels, menopause, and risk of breast cancer: dose-response metaanalysis of prospective studies. Medicine (Baltimore). 2013;92:123-31. doi: 10.1097/ MD.0b013e3182943bc2
12. Dudenkov DV, Yawn BP, Oberhelman SS, et al. Changing incidence of serum 25-hydroxyvitamin D values above 50 ng/mL: A 10-year population-based study. Mayo Clin Proc. 2015;90(5):577-86. doi: 10.1016/j.mayocp.2015.02.012.
13. Ivanov VO, Ivanova SV, Niedzwiecki A. Ascorbate affects proliferation of guinea-pig vascular smooth muscle cells by direct and extracellular matrix-mediated effects. J Mol Cell Cardiol. 1997; 29(12):3293-303.

