Micronutrients in mitigating the adverse health effects of air pollution – part 2
Air pollution is a major environmental risk to human health and well-being. According to WHO reports in 2012, ambient (outdoor) and indoor air pollution was linked to 7 million premature deaths worldwide. Most were attributed to cardiovascular diseases (stroke and ischemic heart disease), chronic obstructive pulmonary disease (COPD), lung cancer, and acute lower respiratory infections in children. Yet, 92% of the world population lives in places that exceed the WHO air quality guidelines. On the other hand, laboratory and clinical studies indicate that a nutritious diet and/or intake of micronutrients with antioxidant, anti-inflammatory, and detoxifying properties, may ameliorate many harmful health effects caused by polluted air.
In Part I of this review published in the Journal of Cellular Medicine and Natural Health (JCM&NH) Issue 4, we discussed cellular and health aspects associated with human exposure to polluted air. Part II presents an overview of potential biological mechanisms underlying the protective effects of vitamins B, C, and E, omega-3 fatty acids, and sulforaphane against air pollutants, as demonstrated in human studies.
Cellular mechanisms involved in protective effects of micronutrients against air pollutants
According to WHO reports, about 90 percent of the world population lives in places that exceed the WHO air quality guidelines.84 This directly translates into higher morbidity and mortality rates—and related to these—loss of quality of life, income, and dramatic increases in healthcare and welfare costs.23,85,86
On the other hand, a growing body of evidence demonstrates that a healthy, nutritious diet is inversely associated with chronic diseases and all-cause mortality.87 Vegetables, fruits, nuts, spices, legumes and cereals rich in micronutrients with antioxidant and anti-inflammatory properties, provide the most health benefits.88,89,90,91
Similarly, epidemiological and experimental studies involving exposure to polluted air corroborate that an intake of vitamins including B, C, and E, omega-3 polyunsaturated fatty acids (PUFAs), and phytochemicals such as sulforaphane, attenuates the deleterious impact of polluted air. The following sections will briefly describe the potential cellular mechanisms underlying the protective effects of natural compounds as investigated in human studies.
1. Protective cellular effects of B-vitamins
The B-vitamins comprise a group of 8 water-soluble vitamins that function as coenzymes in a plethora of anabolic and catabolic biochemical pathways, essential for cellular functions and life .92 For instance, pyridoxal 5‘-phosphate (PLP), a bioactive form of vitamin B6, is an essential cofactor for more than 140 enzyme-catalyzed reactions, and PLP-dependent proteins account for about 4% of total cellular enzymes.93 Collectively, B-vitamins are essential for bio-energy production, amino acid and nucleotide metabolism, DNA/RNA synthesis and repair, and genomic and non-genomic methylation.92 Not surprisingly, low serum levels of B-vitamins such as folate, vitamins B6, and B12 have been linked to multiple chronic diseases as well as increased susceptibility to the harmful effects of air pollution.92,94,96-98
Some interrelated factors underlying the development of chronic diseases include inflammation, a high level of plasma homocysteine, overall epigenetic changes, and gene-specific DNA methylation, all of which directly or indirectly may be related to perturbations in the one-carbon metabolism pathway, which in turn depends on the availability of B-vitamins.99,100,101,102 One-carbon metabolism is a group of biochemical reactions that are involved in transfer of one-carbon groups (e.g., -CH3, -CH2-, -CHO, -CHNH, -CH=) during amino acid and nucleotide metabolism (Fig.1).
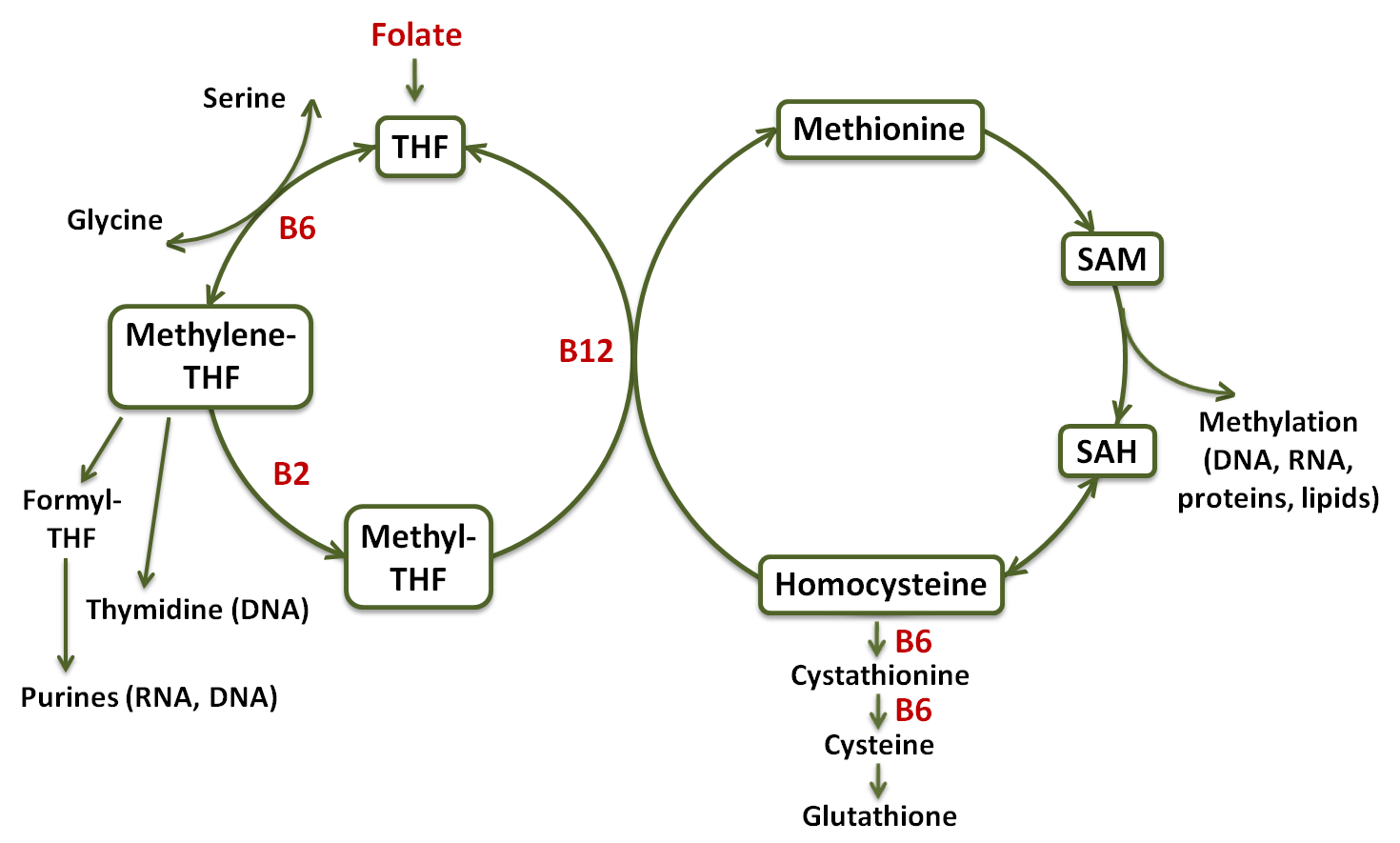
Figure 1 – Diagram of the one-carbon metabolism pathway. Tetrahydrofolate (THF); S-adenosyl methionine (SAM); S-adenosyl homocysteine (SAH) [99, modified].
In short, a carbon unit from amino acid serine or glycine is transferred to tetrahydrofolate (THF), which is a derivative of folate, to form methylene-THF.99 Methylene-THF is either used as such for the synthesis of thymidine, which is then incorporated into DNA, oxidized to formyl-THF, which is utilized for the synthesis of purines, the building blocks of RNA and DNA, or it is reduced to methyl-THF and use to methylate homocysteine to form methionine in a reaction catalyzed by a vitamin B12-dependent methyltransferase. Much of the methionine is converted to S-adenosyl methionine (SAM), a universal methyl donor for the methylation of DNA, RNA, proteins, lipids, hormones, neurotransmitters, and other molecules.99 In addition, homocysteine may be metabolized to cysteine through the sequential action of two vitamin B6-dependent enzymes, and use for glutathione synthesis.
Homocysteine:
Homocysteine, a sulfur-containing amino acid, is a demethylated metabolite of the essential amino acid methionine. Elevated plasma homocysteine levels (hyperhomocysteinemia) is an independent risk factor for cardiovascular diseases (myocardial infarction, heart failure, stroke, and claudication) as well as neurodegenerative diseases and cancer.103,104 Homocysteine is known to promote atherosclerosis through several different mechanisms that adversely affect the function of vascular endothelium and smooth muscle cells (VSMCs).105 Animal studies showed that induction of hyperhomocysteinemia through a diet enriched in methionine but depleted in folate, vitamins B6 and B12, resulted in increased atherosclerotic lesion area and enhanced expression of VCAM-1, matrix metallopeptidase 9 (MMP-9), and receptor for advanced glycation end products (RAGE) in the vasculature. All those effects were counteracted by supplementation with folate, vitamins B6 and B12.106
Also, a trial involving 1000 human subjects showed that air pollution can lead to elevated plasma homocysteine levels.107 This study also revealed that this association can be affected by polymorphism of oxidative stress-related genes.
DNA methylation:
Epigenetic mechanisms of gene regulation such as DNA methylation are recognized as an important link between the genome and the environment.108 DNA methylation, that is the addition of methyl groups to DNA primarily involving SAM, typically represses gene transcription. In this context, elevated serum homocysteine may also reflect a decreased systemic re-methylation capacity and reduced DNA methylation. Indeed, atherosclerosis is associated with an aberrant DNA methylation pattern in vascular tissue and peripheral blood cells, which is believed to be a consequence of a decrease in B-vitamins as factors essential for the synthesis of SAM.109
Also, in the presence of air pollutants, there has been observed marked reduction of DNA methylation in pro-inflammatory, pro-coagulant, and pro-vasoconstriction genes, implying the influence of particulate matter (PM) on cardiovascular biomarkers via epigenetic mechanisms.71,72,110
Detoxification:
The one-carbon pathway incorporating additional vitamin B6-dependent reactions leads to the synthesis of glutathione from homocysteine (Fig. 1). This is notable since observed reductions in heart rate variability (HRV) after PM exposure were associated with functions of the glutathione pathway (GSTM1-null genotype), which suggests the importance of endogenous antioxidant and detoxification mechanisms in mitigating the harmful effects of pollutants.79
All aforementioned mechanisms can provide at least partial explanation of the protective effects of higher B-vitamin intake on autonomic cardiac dysfunction, inflammation and genomic methylation observed in studies in people subjected to air pollution (Table 1) .94,95,111
2. Vitamin C
Vitamin C (ascorbic acid; Fig.2) is a water-soluble vitamin that is synthesized from glucose in the liver of most mammals.112 Humans, non-human primates, and guinea pigs, however, do not have the enzyme gulonolactone oxidase, which is required for vitamin C synthesis, making its consumption mandatory for survival.
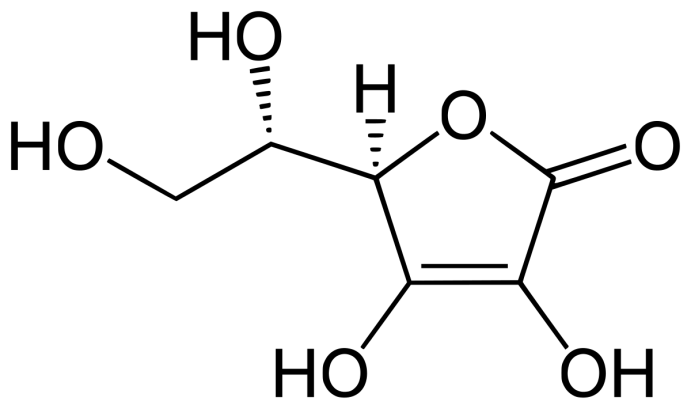
Figure 2 – Chemical structure of vitamin C.
In the body, vitamin C is widely distributed throughout the intracellular and extracellular compartments, where it acts as an electron donor to enzymatic and non-enzymatic reactions. As an electron donor, vitamin C is a potent scavenger for a variety of free radicals and oxidants, including those generated in the body during physiological reactions or leaking from activated neutrophils and macrophages, and for those found in polluted air.112,113
For example, vitamin C is a reducing agent for superoxide radical, hydroxyl radical, peroxyl radicals, reactive sulfur species, and reactive nitrogen species, but also for hypochlorous acid, nitrosamines and other nitrosating compounds, nitrous acid-related compounds, and ozone.112 This may, to a certain extent, explain the improvements in lung function observed in human studies involving vitamin C supplementation during ozone exposure (Table 1). Upon a loss of a second electron, vitamin C forms dehydroascorbate, which can be converted back to ascorbate through various intracellular enzymatic pathways. However, the respiratory tract lining fluid (RTLF) does not appear to have any functional dehydroascorbate reductase activity.112,114,115 Because of that, RTLF acquires ascorbate from cellular sources or the plasma pool, in addition to recycling or salvage mechanisms performed by lung epithelial cells in order to keep an optimal level of this nutrient.113,115
Vitamin C is also essential for cardiovascular health. It regulates collagen synthesis and donates electrons to enzymes, catalyzing hydroxylation of proline and lysine residues in collagen, all essential for maintaining optimum vascular wall integrity and structure.112 In addition, vitamin C inhibits oxidation of LDL cholesterol and affects the regeneration of membrane-bound oxidized vitamin E, by reducing alpha-tocopheroxyl radical to alpha-tocopherol.112,116 It also facilitates the production of nitric oxide by endothelial cells, which in turn promotes vasodilation and blood pressure reduction.117 Furthermore, vitamin C reduces monocyte adhesion to vascular endothelium, thereby protecting against the development of atherosclerosis and it also prevents apoptosis of vascular smooth muscle cells, which helps keep plaque more stable if atherosclerosis has developed.118,119
Table 1 presents clinical studies in which, under increased levels of air pollution, supplementation with vitamin C improved pulmonary function tests and inflammatory and oxidative stress biomarkers in both healthy and asthmatic individuals.
3. Vitamin E
Vitamin E is a collective term for 8 lipid-soluble vitamers i.e., the alpha, beta, gamma and delta classes of tocopherol and tocotrienol (Fig.3).120 Among them, the most extensively studied and also predominantly represented in food and utilized in the body is the d-alpha-tocopherol form of vitamin E. Alpha-tocopherol functions mainly as a potent peroxyl radical scavenger that terminates membrane lipid peroxidation chain reactions.
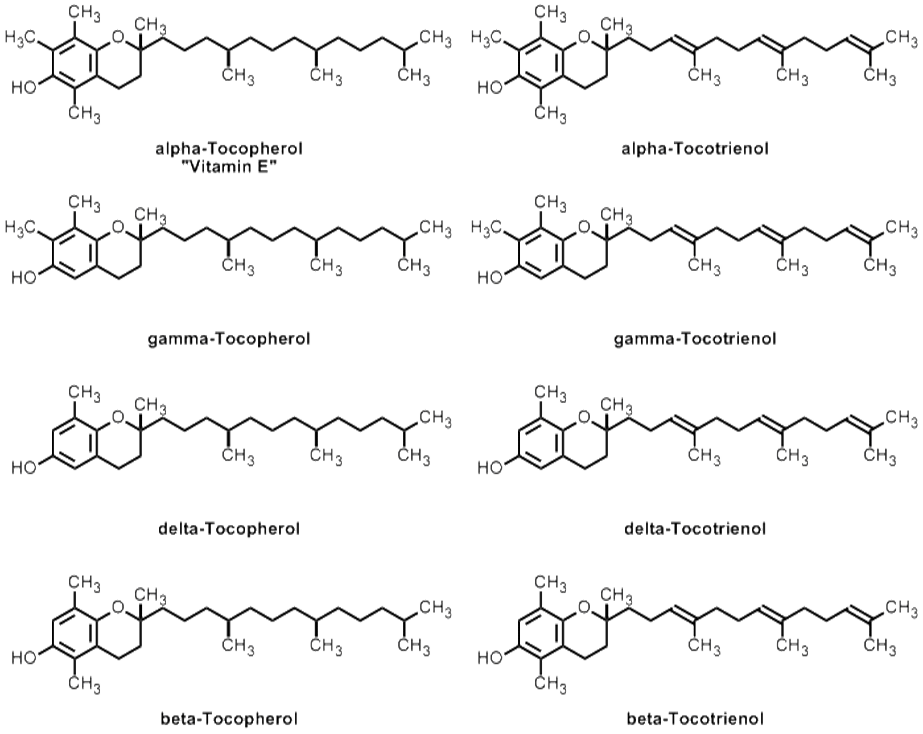
Figure 3 – Chemical structures of vitamin E and other forms of tocopherols and tocotrienols.
It is suggested that the majority of vitamin E effects relate to its antioxidant protection of long-chain polyunsaturated fatty acids, which are important in maintaining the integrity and bioactivity of biological membranes.120 Since these bioactive lipids also function as vital signaling molecules, the change in their levels or their oxidation products are the key cellular events in cellular metabolism, and may also have clinical implications. Pacht et al. reported that young asymptomatic smokers had markedly reduced levels of vitamin E in their alveolar fluid compared to non-smokers (3.1 vs 20.7 ng/ml). Although after 3 weeks of supplementation with 2400 IU/day of vitamin E the level of this vitamin in smoker alveolar fluid showed an increase of up to 9.3 ng/ml, it was still significantly lower relative to non-smokers.121 These shortages could be associated, at least partially, with higher oxidation of vitamin E in the lungs of smokers. Moreover, the alveolar macrophage count in smokers was six-fold higher than in non-smokers, and, as additional investigation revealed, low level of vitamin E in rat‘s lung was associated with increased susceptibility of lung parenchymal cells to alveolar macrophage-induced cytotoxicity, and oxidative lung damage.121
Vitamin E is also indispensable for optimal function of the cardiovascular system, which goes beyond its role in protecting LDL against oxidation.120,122 It has been demonstrated that vitamin E is important in the following: inhibition of pro-inflammatory cytokine production by endothelial cells and immune cells; suppression of adhesion molecules expression on endothelial cells and ligands on monocytes, reducing their adhesive interactions; inhibition of vascular smooth muscle cell proliferation; prevention of platelet aggregation. Vitamin E also modulates cyclooxygenase-2 activity and reduces thromboxane formation, which has vasoconstrictive and platelet aggregation activities, while enhances production of prostacyclin, which has vasodilatory and platelet anti-aggregation properties.120,122 The majority of these effects are believed to be attributed to vitamin E antioxidant activity, however a consensus has not yet been reached.120,123 With respect to its beneficial role during air pollution exposure, several clinical studies showed that vitamin E in combination with vitamin C confers protection against ozone and PM toxicity on respiratory function (Table 1).
4. Omega-3 fatty acids (n-3 FAs)
n-3 FAs are polyunsaturated fatty acids (PUFAs), which are fatty molecules with the first double bond located at the third carbon atom from the end of the carbon chain (Fig. 4). Since they cannot be synthesized in the body, all n-3 FAs must be obtained from the diet or supplementation.124
Following consumption, the n-3 FAs compete with n-6 FAs to be incorporated into all cell membranes, thereby replacing arachidonic acid (AA) (the most important member of the n-6 FA family) for example, for docosahexaenoic acid (DHA) or eicosapentaenoic acid (EPA), the n-3 FAs found in fish oil.125 The composition of PUFAs in cell membrane has an impact on the cell‘s function, partly because these fatty acids represent a reservoir of precursors for eicosanoids such as prostaglandins, leukotrienes, and thromboxanes, which play important signaling or communication roles within and between cells, mediating inflammation, platelet activation, and bronchoconstriction.
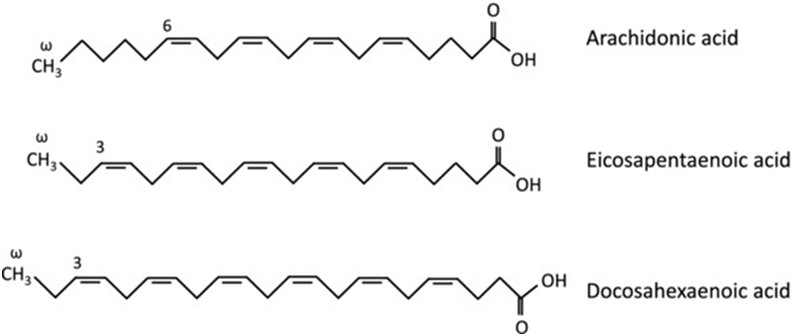
Figure 4 – Chemical structures of omega-3 and omega-6 fatty acids.
Consequently, the substitution of n-3 FAs for n-6 FAs in the membrane decreases the metabolism of arachidonic acid and leads to the formation of less potent inflammatory mediators like prostaglandin E3 (PGE3) and leukotriene 5 (e.g., LTB5 and LTE5), instead of prostaglandin E2 (PGE2) and leukotriene 4 (e.g., LTB4 and LTE4), respectively.113,125
PGE2 has been shown to act on T-lymphocytes to inhibit the production of interferon-gamma (IFN-γ) and other Th1 cytokines without affecting or even stimulating formation of IL-4, a Th2 cytokine [126,127,128]. This modulation of immune response may lead to the development of allergic sensitization, since IL-4 promotes the synthesis of immunoglobulin E, whereas IFN-γ has the opposite effect. Leukotrienes, the other derivatives of AA, are potent bronchoconstrictors (e.g., LTE4), and chemoattractant mediators of inflammation (e.g., LTB4).129 They are also involved in allergic sensitization, vasoconstriction, mucus secretion, and airway remodeling.130 Supplementation of fish oil to pigs increased the porcine alveolar macrophage n-3 FA content and influenced their inflammatory responses by reducing the synthesis of PGE2, LTB4, TNF-alpha, and IL-8.131
In addition to the generation of less potent eicosanoids, EPA and DHA were discovered to be the substrates for a class of anti-inflammatory molecules termed specialized pro-resolving mediators (SPMs).132,133 This class of PUFA metabolites includes n-3 FA-derived resolvins, protectins, and maresins, and n-6 FA-derived lipoxins. SPMs possess potent anti-inflammatory, tissue protecting and tissue healing properties and are actively involved in the clearance and regulation of inflammatory exudates to permit resolution of tissue homeostasis. This finding opens a new avenue for research into the inflammatory process and has an important clinical significance, since inflammation is common to the majority of chronic conditions including pulmonary and cardiovascular diseases.
Another example of the anti-inflammatory action of n-3 FAs is related to a decreased adhesion of immune cells to vascular endothelium. Incorporation of n-3 FAs into endothelial cell phospholipids inhibited the surface expression of vascular cell adhesion molecule-1 (VCAM-1), and subsequent monocytic cell adhesion, suggesting a vasoprotective role of n-3 PUFAs in early atherogenesis, and also in later stages of plaque development and plaque rapture.134,135 Furthermore, n-3 FAs promote endothelial-mediated vasodilation and arterial compliance, by enhancing NO release, contrary to PM-induced inhibition of NO availability.136,137
Intake of n-3 PUFAs has also been associated with increased heart rate variability (HRV) and decreased risk of sudden and non-sudden death from myocardial infarction.138,139,140,141,142 These anti-arrhythmic effects are in part related to modulating activities of n-3 FAs on cardiac ion channels, so that a stronger electrical stimulus is required to elicit an action potential, and the refractory period is considerably prolonged.143,144 n-3 PUFAs inhibit voltage-dependent sodium currents, which initiate action potentials, and calcium currents, which trigger liberation of sarcoplasmic calcium stores and subsequent activation of contractile proteins in myocytes. Another considered anti-arrhythmic mechanism of n-3 PUFAs involves an increase of acetylcholine in the brain, and enhancement of parasympathetic activity while lessening the sympathetic tone.145,146 Altogether, n-3 PUFAs may benefit the cardiovascular system by decreasing the risk of arrhythmias, reducing the risk of thrombosis, improving endothelial function, lowering serum triglycerides, slowing the growth of atherosclerotic plaque, and reducing inflammatory responses147, all contrary to the effects of PM. Results from human studies evaluating the efficacy of fish oil against oxidative stress and declining HRV induced by particulate matter, also showed that supplementation with n-3 FAs augments endogenous antioxidant capacity and prevents HRV decline (Table 1).148,149
5. Sulforaphane
Sulforaphane is a phytochemical within the isothio-cyanate class of organosulfur compounds, derived from cruciferous vegetables such as broccoli, cabbage or cauliflower.150 In plant cells, sulforaphane exists in its precursor form glucoraphanin together with separately compartmentalized enzyme myrosinase. Upon cutting, chewing or otherwise disrupting the plant cell structure, glucoraphanin and myrosinase come into contact, resulting in glucoraphanin hydrolysis to form either bioactive sulforaphane or an inactive sulforaphane nitrile (Fig.5).150,151,152 The shift towards formation of bioactive sulforaphane can be regulated by thermal processing. Steaming for 1-3 min can favor the formation of bioactive sulforaphane by deactivating a heat sensitive non-catalytic cofactor of myrosinase (epithiospecifier protein, ESP) that facilitates the formation of inactive sulforaphane nitrile. Prolonged heating also deactivates myrosinase and therefore decreases the formation of both products.152,153
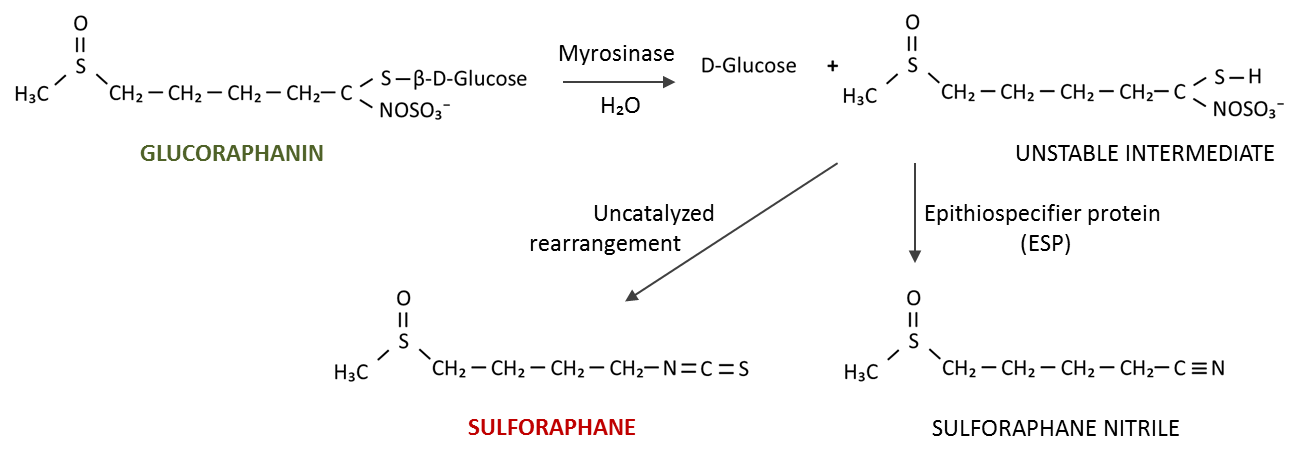
Figure 5 – Formation of sulforaphane from inactive glucoraphanin.
Mechanisms of action:
In the body sulforaphane induces transcription of antioxidant and phase II detoxification genes, which underlie the majority of its beneficial biological effects including cardiovascular, pulmonary, neuroprotective, and anticancer properties.154,155,156,157,158
Sulforaphane belongs to the most potent phytochemical activators of Nrf2 transcription factor, which in turn is a master regulator of basal and inducible expression of a battery of both antioxidant genes that preserve cellular homeostasis, and detoxification genes that process and eliminate toxins and carcinogens before they can cause damage.150,158,160,161 Although the exact mechanism is still unclear, it appears that sulforaphane disrupts the cytoplasmic complex formed between the actin-bound protein Keap1 and the transcription factor Nrf2. This releases Nrf2 for translocation into the nucleus, where it activates the antioxidant response element (ARE)-dependent genes.159 In addition, sulforaphane might affect the activity of intracellular kinases to phosphorylate Nrf2 protein, which modulates the Nrf2 stability and/or dictates its nucleocytoplasmic trafficking.159
It has been demonstrated in vitro, in vivo and in human studies, that sulforaphane can induce several Nrf2-regulated genes including superoxide dismutase (SOD), catalase (CAT), hemoxygenase-1 (HO-1), glutathione peroxidase (GPx), glutathione reductase (GR), glutathione S-transferase (GST), NAD(P)H:quinone oxidoreductase 1 (NQO1), gamma-glutamylcysteine synthetase (GSC), glucuronosyltransferases (UGTs), epoxide hydrolase as well as NADPH-regenerating enzymes.158,160,161,166 Up-regulation of those genes enhances the endogenous antioxidant defense capacity and is critical during elevated oxidative stress caused by disease (e.g., asthma, allergy, COPD), air pollutants (DEP, tobacco smoke), and even allergens such as ragweed pollen. This allergen contains NADPH oxidases, which can generate ROS, followed by robust allergic airway inflammation in sensitized subjects.162,163,164
Furthermore, sulforphane affects phase II detoxification enzymes which facilitate the removal of a variety of xenobiotics from the body via conjugation reactions (e.g., with glutathione).165 The efficacy of sulforaphane to induce Nrf2-regulated genes and enhance the urinary excretion of some airborne pollutants or reduce the inflammatory effects of oxidative stress in upper airways, has been demonstrated in several human studies (Table 1).166,167,168 Moreover, an interest in natural Nrf2 inducers is growing among clinicians and currently there are 49 studies registered on www.clinicaltrials.gov exploring the potential therapeutic benefits of sulforphane with respect to a wide spectrum of neurological, oncological, respiratory, and cardiovascular diseases.
Micronutrients against air pollutants: main outcomes from the human studies
In recent decades, there has been a growing interest in searching for natural compounds as alternative or complementary solutions to many health problems. Several studies in both healthy and asthmatic subjects of all ages, investigated the efficacy of antioxidant vitamins C and E in counteracting adverse respiratory symptoms after exposure to high ground-level ozone. The results demonstrated significant improvements in lung function in those who underwent supplementation.170,171,172,173,174,175 Vitamins C and E were also found to confer protective effects against PM-induced oxidative damage associated with airborne contamination from coal burning from an electric-power plant.176
Furthermore, omega-3 FAs and B vitamins were found to attenuate cardiac autonomic dysfunction related to PM exposure.94,139,148 As such, supplementation with omega-3 FAs enhanced HRV and antioxidant capacity as well as prevented an increase in triglyceride and very low density lipoprotein (VLDL) concentrations associated with ultrafine particle (UFP) inhalation.139,148,149
Intake of B vitamins also protected against PM2.5-induced depletion of mitochondrial DNA content and methylation changes in genes involved in mitochondrial energy metabolism.95
In another study, administration of sulforaphane-rich broccoli sprout extract (a potent Nrf2 inductor) for 4 days before the nasal diesel exhaust particle challenge, reduced total white blood cell count in nasal lavage fluid by 54% compared to control.167
Table 1 provides more detail on the above-described human studies on the benefits of micronutrient supplementation during air pollution exposure.
Table 1 – Human studies involving micronutrient supplementation during air pollution exposure.
| 94OBJECTIVE To determine whether B vitamin supplementation mitigates PM2.5 effects on cardiac autonomic dysfunction and inflammation. DESIGN Single-blind, placebo-controlled, crossover study. PARTICIPANTS 10 healthy adults. NUTRIENT INTERVENTION Folic acid (2.5 mg/d), vitamin B6 (50 mg/d), and vitamin B12 (1mg/d). PRIMARY RESULTS PM2.5 exposure increased HR, LF-HRV, total WBC count, and lymphocyte count. B vitamin supplementation attenuated PM2.5 effect on HR by 150%, LF-HRV by 90%, total WBC count by 139%, and lymphocyte count by 106%. CONCLUSION B vitamin supplementation can diminish the acute effects of PM2.5 on cardiac autonomic dysfunction and inflammatory markers. |
| 95OBJECTIVE To determine whether B vitamin supplementation averts PM2.5 induced DNA methylation changes implicated in inflammation and oxidative stress. DESIGN Single-blind, placebo-controlled, crossover study. PARTICIPANTS 10 healthy adults. NUTRIENT INTERVENTION Folic acid (2.5 mg/d), vitamin B6 (50 mg/d), and vitamin B12 (1mg/d). PRIMARY RESULTS PM2.5 induced methylation changes in genes involved in mitochondrial oxidative energy metabolism. B vitamin supplementation prevented these changes. Likewise, PM2.5 depleted 11.1% of mitochondrial DNA content compared with sham, and B vitamin supplementation attenuated the PM2.5 effect by 102%. CONCLUSION The individual-level prevention may be used to complement regulations and control potential mechanistic pathways underlying the adverse PM2.5 effects. |
| 169OBJECTIVE To determine the effect of vitamin C on NO2-induced airway hyperresponsiveness (assessed with methacholine aerosol). DESIGN Randomized, double-blind, placebocontrolled study. PARTICIPANTS 11 normal subjects. NUTRIENT INTERVENTION Vitamin C (4×500 mg/d). PRIMARY RESULTS NO2 exposure resulted in significant enhancement of airway responsiveness to methacholine in the placebo group. Supplementation with vitamin C completely prevented alternation in airway responsiveness. CONCLUSION Vitamin C prevented airway hyperresponsiveness induced by NO2. |
| 170OBJECTIVE To evaluate whether acute effects of O3, NO2, and PM10 could be attenuated by antioxidant supplementation. DESIGN Randomized, double-blind, placebo-controlled study. PARTICIPANTS 158 children with asthma. NUTRIENT INTERVENTION Vitamin C (250 mg/d) and vitamin E (50 mg/d). PRIMARY RESULTS O3 exposure significantly reduced lung functions in placebo group but not in the supplement group. There were no statistically significant changes in pulmonary function parameters upon NO2 and PM10 exposure. CONCLUSION Supplementation with antioxidants might modulate the impact of ozone exposure on the small airways of children with moderate to severe asthma. |
| 171OBJECTIVE To investigate the impact of antioxidant supplementation on the nasal inflammatory response to O3 exposure. DESIGN Randomized, double-blind, placebo-controlled study. PARTICIPANTS 117 children with asthma. NUTRIENT INTERVENTION Vitamin C (250 mg/d) and vitamin E (50 mg/d). PRIMARY RESULTS O3 exposure increased IL-6 and IL-8 in the nasal lavage in the placebo group but not in the supplement group. CONCLUSION Supplementation of vitamins C and E above the minimum dietary requirement in asthmatic children with a low intake of vitamin E might provide some protection against the nasal acute inflammatory response to ozone. |
| 172OBJECTIVE To evaluate the effects of dietary antioxidants on O3-induced bronchial hyperresponsiveness (assessed with 10-min SO2 inhalation challenge). DESIGN Double-blind crossover study. PARTICIPANTS 17 adults with asthma. NUTRIENT INTERVENTION Vitamin C (500 mg/d) and vitamin E (400 IU/d). PRIMARY RESULTS Supplementation with vitamins C and E improved spirometric parameters and reduced pulmonary responsiveness upon SO2 challenge. CONCLUSION Vitamins C and E may benefit asthmatic adults exposed to air pollutants. |
| 173OBJECTIVE To determine whether antioxidants can influence human susceptibility to O3- induced changes in lung function and airway inflammation. DESIGN Randomized, placebo-controlled study. PARTICIPANTS 31 healthy nonsmoking adults. NUTRIENT INTERVENTION Vitamin C (250 mg/d), vitamin E (50 IU/d) and vegetable cocktail (12 oz/d). PRIMARY RESULTS Pulmonary function testing showed that O3-induced reductions in spirometric parameters i.e., FEV(1) and FVC were 30% and 24% smaller respectively in the supplemented cohort. In contrast, there was no apparent effect in the supplement group on the severity of the inflammation provoked by O3 exposure. CONCLUSION Vitamins C and E may protect against ozone-induced pulmonary function decrements in humans. |
| 174OBJECTIVE To investigate whether the acute effects of O3 on lung function could be modulated by antioxidant vitamin supplementation. DESIGN Randomized, double-blind, placebocontrolled study PARTICIPANTS 38 cyclists. NUTRIENT INTERVENTION Vitamin C (500 mg/d) and vitamin E (100 mg/d). PRIMARY RESULTS A difference in ozone exposure of 100 μg/m3 decreased FEV1 and FVC by 95 ml and 125 ml respectively in placebo group and by 1 ml and 42 ml respectively in the vitamin group. CONCLUSION Supplementation with vitamins C and E confers partial protection against the acute effects of ozone on lung functions in cyclists. |
| 175OBJECTIVE To evaluate whether acute effects of O3 on lung functions could be attenuated by antioxidant vitamin supplementation. DESIGN Randomized, double-blind, placebocontrolled, crossover study PARTICIPANTS 47 street workers. NUTRIENT INTERVENTION Vitamin C (650 mg/d), vitamin E (75 mg/d) and beta-carotene (15 mg/d). PRIMARY RESULTS O3 levels were inversely associated with spirometric parameters in the placebo group but not in the supplement group. It was estimated that for a daily mean O3 exposure of 70 ppb, the supplement effect would correspond to an attenuation of lung function decrements of 3.4% for FEV1 and 6.1% for FEF25-75, whereas during the same exposure for two consecutive days, this effect would reach 7.4% and 16.6% for FEV1 and FEF25-75 respectively. CONCLUSION Supplementation above the recommended allowance may provide additional protection against the acute effects of high ozone exposure on lung functions. |
| 176OBJECTIVE To better understand the relations between PM exposure derived from a coalburning electric power plant and the oxidative damage in subjects directly or indirectly exposed to airborne contamination before and after 6 months of supplementation in comparison to non-exposed subjects (control group). DESIGN Randomized, controlled study PARTICIPANTS 80 adults (directly exposed: workers handling mineral coal, n=20; indirectly exposed: office workers of the power plant, n=20; residents living within 2 km, n=20; control subjects living 100 km from emission, n=20). NUTRIENT INTERVENTION Vitamin C (500 mg/d) and vitamin E (800 mg/d). PRIMARY RESULTS Before supplementation, levels of oxidative stress biomarkers (TBARS and PC) were significantly increased, levels of GSH and vitamin E were decreased, while the activities of SOD and CAT were increased in the workers’ group and GST was increased in all groups in compared to controls. After the antioxidant supplementation essentially all these biomarkers were normalized to control levels. CONCLUSION Vitamins C and E conferred protective effects against the oxidative insults associated with airborne contamination derived from coal burning of an electric-power plant. |
| 148OBJECTIVE To evaluate the effect of supplementation with omega-3 PUFAs on the reduction of HRV associated with PM2.5 exposure. DESIGN Randomized, double-blind trial. PARTICIPANTS 50 nursing home residents older than 60 years. NUTRIENT INTERVENTION Fish oil (2 g/d) or soy oil (2 g/d). PRIMARY RESULTS In the group receiving fish oil there was a 54% reduction in HRV-HF per 1SD (8 μg/m3) of PM2.5 before supplementation phase and 7% reduction in HRV-HF per 1SD of PM2.5 during the supplementation phase. CONCLUSION Fish oil supplementation prevented HRV decline related to PM2.5 exposure and was significantly better than soy oil supplementation. |
| 149OBJECTIVE To evaluate whether omega-3 PUFA supplementation could protect against the cardiac alternations linked to PM exposure by measuring biomarkers of response to oxidative stimuli. DESIGN Randomized, double-blind, controlled study. PARTICIPANTS 52 nursing home residents older than 60 years. NUTRIENT INTERVENTION Fish oil (2 g/d) or soy oil (2 g/d). PRIMARY RESULTS Supplementation with either fish oil or soy oil increased SOD activity and GSH levels. In addition, fish oil reduced lipoperoxidation products in plasma. CONCLUSION Omega-3 PUFAs may modulate oxidative response to PM2.5 exposure. |
| 139OBJECTIVE To evaluate the efficacy of fish oil supplements in attenuating adverse cardiac effects of exposure to concentrated ambient fine and ultrafine particulate matter (CAP). DESIGN Randomized, double-blind, controlled study. PARTICIPANTS 29 healthy middle-aged adults. NUTRIENT INTERVENTION Fish oil (3 g/d) or olive oil (3 g/d). PRIMARY RESULTS Fish oil supplementation attenuated CAP-induced reductions in HF/LF ratio, as well as elevations in normalized LFHRV and prolongation of the QT interval corrected for HR (QTc). VLDL and TG concentrations increased significantly immediately after exposure to CAP in the olive oil group but not in the fish oil group. CONCLUSION Omega-3 PUFAs offer protection against the adverse cardiac and lipid effects associated with air pollution exposure. |
| 168OBJECTIVE To evaluate the magnitude and duration of pharmacodynamic action of a beverage enriched with glucoraphanin (GR) and sulforaphane (SF) from broccoli sprouts. DESIGN Randomized, placebo-controlled study. PARTICIPANTS 291 adults in good general health. NUTRIENT INTERVENTION Beverage providing 600 μmol of GR and 40 μmol of SF daily. PRIMARY RESULTS Rapid and sustained increases in the levels of urinary excretion of the glutathione-derived conjugates of benzene (61%) and acrolein (23%), but not crotonaldehyde, were found in subjects receiving broccoli sprout beverage compared with placebo. CONCLUSION Intervention with broccoli sprouts enhances the detoxification of some airborne pollutants and may provide a frugal means to attenuate their associated longterm health risks. |
| 167OBJECTIVE To determine whether the administration of a standardized broccoli sprout extract (BSE) could be used to suppress the nasal inflammatory response in human subjects challenged with 300 μg of an aqueous diesel exhaust particle (DEP) suspension (equivalent to daily PM exposure levels on a Los Angeles freeway). DESIGN Controlled study. PARTICIPANTS 29 healthy adults. NUTRIENT INTERVENTION BSE, providing 100 μmol of sulforaphane, in mango juice daily. PRIMARY RESULTS The nasal DEP challenge significantly increased WBC count in nasal lavage fluid. When DEP challenge was preceded by 4 days of daily BSE administration, total WBC counts decreased by 54%. CONCLUSION The study demonstrates the potential preventive and therapeutic potential of broccoli or broccoli sprouts rich in glucoraphanin for reducing the impact of particulate pollution on allergic disease and asthma. |
Abbreviations: CAT, catalase; FEF25-75, forced expiratory flow between 25-75% of the FVC; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; GSH, glutathione; GST, glutathione S-transferase; HR, heart rate; HRV, heart rate variability; HF-HRV, high frequency HRV; IL, interleukin; LF-HRV, low frequency HRV; PC, protein carbonyls; SD, standard deviation; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substances; TG, triglycerides; VLDL, very low density lipoprotein; WBC, white blood cell.
Conclusion
Numerous studies ranging from epidemiological through clinical to toxicological evaluations provide persuasive evidence that air pollution is a major contributor to cardiopulmonary morbidity and mortality worldwide. Scientific findings also indicate that gaseous or particulate pollutants primarily exert their effects through oxidative stress and inflammatory pathways. Additionally, individual usceptibility — largely determined by age, underlying medical conditions, genetic background and nutrition — is an important factor that enhances morbidity and mortality associated with air pollution exposure.
Various laboratory and clinical studies with antioxidant, anti-inflammatory and detoxifying micronutrients provide better understanding of the biochemical mechanisms underlying the improvements in clinical symptoms observed after supplementation. It should be noted that beneficial results were seen with application of vitamins B, C and E above the recommended doses (RDA), which implies that low dosages of these vitamins hinder their therapeutic potential. More studies are needed to further explore the efficacy not only of those nutrients already presented, but also other naturallyderived
compounds as well as their possible/potential synergistic combinations, in order to establish efficient, non-toxic, and cost-effective treatments applicable to various health concerns associated with air pollution insult.
References
84. 7 million premature deaths annually linked to air pollution. World Health Organization website. www.who.int/mediacentre/news/releases/2014/airpollution/en/. Accessed May 25, 2017.
85. WHO releases country estimates on air pollution exposure and health impact. World Health Organization. www.who.int/mediacentre/news/releases/2016/air-pollutionestimates/en/. Accessed May 25, 2017.
86. Air pollution deaths cost global economy US$225 billion. The World Bank Website. www.worldbank.org/en/news/press-release/2016/09/08/air-pollution-deaths-cost-globaleconomy-225-billion. Accessed May 25, 2017.
87. Chiuve SE, Sampson L, Willett WC. The association between a nutritional quality index and risk of chronic disease. Am J Prev Med. 2011; 40(5): 505-13.
88. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronicdiseases. Exp Biol Med (Maywood). 2008; 233(6): 674-88.
89. Landete JM. Dietary intake of natural antioxidants: vitamins and polyphenols. Crit Rev Food Sci Nutr. 2013; 53(7): 706-21.
90. Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012; 54 Suppl: S29-37.
91. Hoeft B, Weber P, Eggersdorfer M. Micronutrients – a global perspective on intake, health benefits and economics. Int J Vitam Nutr Res. 2012; 82(5): 316-20.
92. Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy- a review. Nutrients. 2016; 8(2); 68.
93. Stover PJ, Field MS. Vitamin B-6. Adv Nutr. 2015; 6(1): 132-3.
94. Zhong J, Trevisi L, Urch B, et al. B-vitamin supplementation mitigates effects of fine particles on cardiac autonomic dysfunction and inflammation: a pilot human intervention trial. Sci Rep. 2017; 7: 45322.
95. Zhong J, Karlsson O, Wang G, et al. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc Natl Acad Sci USA. 2017; 114(13): 3503-3508.
96. Kolling J, Scherer EB, da Cunha AA, da Cunha MJ, Wyse AT. Homocysteine induces oxidative-nitrative stress in heart of rats: prevention by folic acid. Cardiovasc Toxicol. 2011; 11(1): 67-73.
97. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010; 303(23): 2377-85.
98. Fimognari FL, Loffredo L, Di Simone S, et al. Hyperhomocysteinaemia and poor vitamin B status in chronic obstructive pulmonary disease. Nutr Metab Cardiovasc Dis. 2009; 19(9): 654-9.
99. Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002; 6(1); 39-42.
100. Chi GC, Liu Y, MacDonald JW, et al. Long-term outdoor air pollution and DNA methylation in circulating monocytes: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health. 2016; 15(1): 119.
101. Fiorito G, Guarrera S, Valle C, et al. B-vitamins intake, DNAmethylation of One Carbon Metabolism and homocysteine pathway genes and myocardial infarction risk: the EPICOR study. Nutr Metab Cardiovasc Dis. 2014; 24(5): 483-8.
102. Abbenhardt C, Miller JW, Song X, et al. Biomarkers of one-carbon metabolism are associated with biomarkers of inflammation in women. J Nutr. 2014; 144(5): 714-21.
103. Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015; 14: 6.
104. Wu LL, Wu JT. Hyperhomocysteinemia is a risk factor for cancer and a new potential tumor marker. Clin Chim Acta. 2002; 322(1-2): 21-8.
105. Zhang S, Bai YY, Luo LM, Xiao WK, Wu HM, Ye P. Association between serum homocysteine and arterial stiffness in elderly: a community-based study. J Geriatr Cardiol. 2014; 11(1): 32-8.
106. Hofmann MA, Lalla E, Lu Y et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001; 107(6): 675-83.
107. Ren C, Park SK, Vokonas PS, et al. Air pollution and homocysteine: more evidence that oxidative stress-related genes modify effects of particulate air pollution. Epidemiology. 2010; 21(2): 198-206.
108. Madrigano J, Baccarelli A, Mittleman MA, et al. Prolonged exposure to particulate pollution, genes associated with glutathione pathways, and DNA methylation in a cohort of older men. Environ Health Perspect. 2011; 119(7): 977-82.
109. Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr. 2005; 135(1): 5-8.
110. Bind MA, Lepeule J, Zanobetti A, et al. Air pollution and gene-specific methylation in the Normative Aging Study: association, effect modification, and mediation analysis. Epigenetics. 2014; 9(3): 448-58.
111. Baccarelli A, Cassano PA, Litonjua A, et al. Cardiac autonomic dysfunction: effects from particulate air pollution and protection by dietary methyl nutrients and metabolic polymorphisms. Circulation. 2008; 117(14): 1802-9.
112. Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003; 22(1): 18-35.
113. Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: a review. Eur Respir J. 2008; 31(1): 179-97.
114. van der Vliet A, O’Neill CA, Cross CE, et al. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol. 1999; 276(2 Pt 1): L289-96.
115. Larsson N, Rankin GD, Bicer EM, et al. Identification of vitamin C transporters in the human airways: a cross-sectional in vivo study. BMJ Open. 2015; 5(4): e006979.
116. Niki E. Interaction of ascorbate and alpha-tocopherol. Ann N Y Acad Sci. 1987; 498: 186-99.
117. d’Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2003; 92(1): 88-95.
118. Siow RC, Richards JP, Pedley KC, Leake DS, Mann GE. Vitamin C protects human vascular smooth muscle cells against apoptosis induced by moderately oxidized LDL containing high levels of lipid hydroperoxides. Arterioscler Thromb Vasc Biol. 1999; 19(10): 2387-94.
119. Weber C, Erl W, Weber K, Weber PC. Increased adhesiveness of isolated monocytes to endothelium is prevented by vitamin C intake in smokers. Circulation. 1996; 93(8): 1488-92.
120. Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007; 43(1): 4-15.
121. Pacht ER, Kaseki H, Mohammed JR, Cornwell DG, Davis WB. Deficiency of vitamin E in the alveolar fluid of cigarette smokers. Influence on alveolar macrophage cytotoxicity. J Clin Invest. 1986; 77(3): 789-96.
122. Meydani M. Vitamin E and atherosclerosis: beyond prevention of LDL oxidation. J Nutr. 2001; 131(2): 366S-8S.
123. Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005; 25: 151-74.
124. Surette ME. The science behind dietary omega-3 fatty acids. CMAJ. 2008; 178(2): 177-80.
125. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006; 83(6 Suppl): 1505S-1519S.
126. Snijdewint FG, Kaliński P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993; 150(12): 5321-9.
127. Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991; 146(1): 108-13.
128. Fedyk ER, Phipps RP. Prostaglandin E2 receptors of the EP2 and EP4 subtypes regulate activation and differentiation of mouse B lymphocytes to IgE-secreting cells. Proc Natl Acad Sci USA. 1996; 93(20): 10978-83.
129. Sharma JN, Mohammed LA. The role of leukotrienes in the pathophysiology of inflammatory disorders: is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology. 2006; 14(1-2): 10-6.
130. Hallstrand TS, Henderson WR Jr. An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol. 2010; 10(1): 60-6.
131. Møller S, Lauridsen C. Dietary fatty acid composition rather than vitamin E supplementation influence ex vivo cytokine and eicosanoid response of porcine alveolar macrophages. Cytokine. 2006; 35(1-2): 6-12.
132. Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol. 2016; 785: 144-155.
133. Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim Biophys Acta. 2010; 1801(12): 1260-73.
134. Weber C, Erl W, Pietsch A, Danesch U, Weber PC. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 1995; 15(5): 622-8.
135. De Caterina R, Massaro M. Omega-3 fatty acids and the regulation of expression of endothelial pro-atherogenic and pro-inflammatory genes. J Membr Biol. 2005; 206(2): 103-16.
136. McVeigh GE, Brennan GM, Johnston GD, et al. Dietary fish oil augments nitric oxide production or release in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993; 36(1): 33-8.
137. Muto E, Hayashi T, Yamada K, Esaki T, Sagai M, Iguchi A. Endothelial-constitutive nitric oxide synthase exists in airways and diesel exhaust particles inhibit the effect of nitric oxide. Life Sci. 1996; 59(18): 1563-70.
138. Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002; 105(16): 1897-903.
139. Tong H, Rappold AG, Diaz-Sanchez D, et al. Omega-3 fatty acid supplementation appears to attenuate particulate air pollution-induced cardiac effects and lipid changes in healthy middle-aged adults. Environ Health Perspect. 2012; 120(7): 952-7.
140. Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009; 54(7): 585-94.
141. Harris WS, Kris-Etherton PM, Harris KA. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr Atheroscler Rep. 2008; 10(6): 503-9.
142. Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005; 112(13): 1945-52.
143. Xiao YF, Sigg DC, Leaf A. The antiarrhythmic effect of n-3 polyunsaturated fatty acids: modulation of cardiac ion channels as a potential mechanism. J Membr Biol. 2005; 206(2):141-54.
144. Leaf A. The electrophysiologic basis for the antiarrhythmic and anticonvulsant effects of n-3 polyunsaturated fatty acids: heart and brain. Lipids. 2001; 36 Suppl: S107-10.
145. Neki NS, Singh RB, Rastogi SS. How brain influences neuro-cardiovascular dysfunction. J Assoc Physicians India. 2004; 52: 223-30.
146. Christensen JH, Christensen MS, Dyerberg J, Schmidt EB. Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n-3 fatty acids. Am J Clin Nutr. 1999; 70(3): 331-7.
147. Kris-Etheron PM, Harris WS, Appel LJ. Omega-3 fatty acids and cardiovascular disease. New recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003; 23: 151-152.
148. Romieu I, Téllez-Rojo MM, Lazo M, et al. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med. 2005; 172(12): 1534-40.
149. Romieu I, Garcia-Esteban R, Sunyer J, et al. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM(2.5). Environ Health Perspect. 2008; 116(9): 1237-42.
150. Houghton CA, Fassett RG, Coombes JS. Sulforaphane and other nutrigenomic Nrf2 activators: Can the clinician’s expectation be matched by the reality? Oxid Med Cell Longev. 2016; 2016: 7857186.
151. Matusheski NV, Jeffery EH. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J Agric Food Chem. 2001; 49(12): 5743-9.
152. Wang GC, Farnham M, Jeffery EH. Impact of thermal processing on sulforaphane yield from broccoli (Brassica oleracea L. ssp. italica). J Agric Food Chem. 2012; 60(27): 6743-8.
153. Matusheski NV, Juvik JA, Jeffery EH. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry. 2004; 65(9): 1273-81.
154. Xu Z, Wang S, Ji H, et al. Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Sci Rep. 2016; 6: 30252
155. Harvey CJ, Thimmulappa RK, Sethi S, et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med. 2011; 3(78): 78ra32.
156. Munday R, Mhawech-Fauceglia P, Munday CM, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008; 68(5): 1593-600.
157. Tarozzi A, Angeloni C, Malaguti M, Morroni F, Hrelia S, Hrelia P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid Med Cell Longev. 2013; 2013:
158. Zhu H, Jia Z, Strobl JS, Ehrich M, Misra HP, Li Y. Potent induction of total cellular and mitochondrial antioxidants and phase 2 enzymes by cruciferous sulforaphane in rat aortic smooth muscle cells: cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol. 2008; 8(3): 115-25.
159. Keum YS. Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: implications of posttranslational modifications. Ann N Y Acad Sci. 2011; 1229:184-9.
160. Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002; 62(18): 5196-203.
161. Stefanson AL, Bakovic M. Dietary regulation of Keap1/Nrf2/ARE pathway: focus on plant-derived compounds and trace minerals. Nutrients. 2014; 6(9): 3777-801.
162. Boldogh I, Bacsi A, Choudhury BK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005; 115(8): 2169-79.
163. Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol. 2005; 115(2): 221-8; quiz 229.
164. Riedl MA, Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol. 2008; 8(1): 49-56.
165. Delfino RJ, Staimer N, Vaziri ND. Air pollution and circulating biomarkers of oxidative stress. Air Qual Atmos Health. 2011; 4(1): 37-52.
166. Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009; 130(3): 244-51.
167. Heber D, Li Z, Garcia-Lloret M, et al. Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food Funct. 2014; 5(1): 35-41.
168. Egner PA, Chen JG, Zarth AT, et al. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila). 2014; 7(8): 813-823.
169. Mohsenin V. Effect of vitamin C on NO2-induced airway hyperresponsiveness in normal subjects. A randomized double-blind experiment. Am Rev Respir Dis. 1987; 136(6): 1408-11.
170. Romieu I, Sienra-Monge JJ, Ramírez-Aguilar M, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002; 166(5): 703-9.
171. Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, et al. Antioxidant supplementation and nasal inflammatory responses among young asthmatics exposed to high levels of ozone. Clin Exp Immunol. 2004; 138(2): 317-22.
172. Trenga CA, Koenig JQ, Williams PV. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Arch Environ Health. 2001; 56(3): 242-9.
173. Samet JM, Hatch GE, Horstman D, et al. Effect of antioxidant supplementation on ozone-induced lung injury in human subjects. Am J Respir Crit Care Med. 2001; 164(5): 819-25.
174. Grievink L, Zijlstra AG, Ke X, Brunekreef B. Double-blind intervention trial on modulation of ozone effects on pulmonary function by antioxidant supplements. Am J Epidemiol. 1999; 149(4): 306-14.
175. Romieu I, Meneses F, Ramirez M, et al. Antioxidant supplementation and respiratory functions among workers exposed to high levels of ozone. Am J Respir Crit Care Med. 1998; 158(1): 226-32
176. Possamai FP, Júnior SÁ, Parisotto EB, et al. Antioxidant intervention compensates oxidative stress in blood of subjects exposed to emissions from a coal electric-power plant in South Brazil. Environ Toxicol Pharmacol. 2010; 30(2): 175-80.

Figure 1 – Diagram of the one-carbon metabolism pathway. Tetrahydrofolate (THF); S-adenosyl methionine (SAM); S-adenosyl homocysteine (SAH) [99, modified].

Figure 2 – Chemical structure of vitamin C.

Figure 3 – Chemical structures of vitamin E and other forms of tocopherols and tocotrienols.

Figure 4 – Chemical structures of omega-3 and omega-6 fatty acids.

Figure 5 – Formation of sulforaphane from inactive glucoraphanin.
84. 7 million premature deaths annually linked to air pollution. World Health Organization website. www.who.int/mediacentre/news/releases/2014/airpollution/en/. Accessed May 25, 2017.
85. WHO releases country estimates on air pollution exposure and health impact. World Health Organization. www.who.int/mediacentre/news/releases/2016/air-pollutionestimates/en/. Accessed May 25, 2017.
86. Air pollution deaths cost global economy US$225 billion. The World Bank Website. www.worldbank.org/en/news/press-release/2016/09/08/air-pollution-deaths-cost-globaleconomy-225-billion. Accessed May 25, 2017.
87. Chiuve SE, Sampson L, Willett WC. The association between a nutritional quality index and risk of chronic disease. Am J Prev Med. 2011; 40(5): 505-13.
88. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronicdiseases. Exp Biol Med (Maywood). 2008; 233(6): 674-88.
89. Landete JM. Dietary intake of natural antioxidants: vitamins and polyphenols. Crit Rev Food Sci Nutr. 2013; 53(7): 706-21.
90. Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012; 54 Suppl: S29-37.
91. Hoeft B, Weber P, Eggersdorfer M. Micronutrients – a global perspective on intake, health benefits and economics. Int J Vitam Nutr Res. 2012; 82(5): 316-20.
92. Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy- a review. Nutrients. 2016; 8(2); 68.
93. Stover PJ, Field MS. Vitamin B-6. Adv Nutr. 2015; 6(1): 132-3.
94. Zhong J, Trevisi L, Urch B, et al. B-vitamin supplementation mitigates effects of fine particles on cardiac autonomic dysfunction and inflammation: a pilot human intervention trial. Sci Rep. 2017; 7: 45322.
95. Zhong J, Karlsson O, Wang G, et al. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc Natl Acad Sci USA. 2017; 114(13): 3503-3508.
96. Kolling J, Scherer EB, da Cunha AA, da Cunha MJ, Wyse AT. Homocysteine induces oxidative-nitrative stress in heart of rats: prevention by folic acid. Cardiovasc Toxicol. 2011; 11(1): 67-73.
97. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010; 303(23): 2377-85.
98. Fimognari FL, Loffredo L, Di Simone S, et al. Hyperhomocysteinaemia and poor vitamin B status in chronic obstructive pulmonary disease. Nutr Metab Cardiovasc Dis. 2009; 19(9): 654-9.
99. Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002; 6(1); 39-42.
100. Chi GC, Liu Y, MacDonald JW, et al. Long-term outdoor air pollution and DNA methylation in circulating monocytes: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health. 2016; 15(1): 119.
101. Fiorito G, Guarrera S, Valle C, et al. B-vitamins intake, DNAmethylation of One Carbon Metabolism and homocysteine pathway genes and myocardial infarction risk: the EPICOR study. Nutr Metab Cardiovasc Dis. 2014; 24(5): 483-8.
102. Abbenhardt C, Miller JW, Song X, et al. Biomarkers of one-carbon metabolism are associated with biomarkers of inflammation in women. J Nutr. 2014; 144(5): 714-21.
103. Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015; 14: 6.
104. Wu LL, Wu JT. Hyperhomocysteinemia is a risk factor for cancer and a new potential tumor marker. Clin Chim Acta. 2002; 322(1-2): 21-8.
105. Zhang S, Bai YY, Luo LM, Xiao WK, Wu HM, Ye P. Association between serum homocysteine and arterial stiffness in elderly: a community-based study. J Geriatr Cardiol. 2014; 11(1): 32-8.
106. Hofmann MA, Lalla E, Lu Y et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001; 107(6): 675-83.
107. Ren C, Park SK, Vokonas PS, et al. Air pollution and homocysteine: more evidence that oxidative stress-related genes modify effects of particulate air pollution. Epidemiology. 2010; 21(2): 198-206.
108. Madrigano J, Baccarelli A, Mittleman MA, et al. Prolonged exposure to particulate pollution, genes associated with glutathione pathways, and DNA methylation in a cohort of older men. Environ Health Perspect. 2011; 119(7): 977-82.
109. Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr. 2005; 135(1): 5-8.
110. Bind MA, Lepeule J, Zanobetti A, et al. Air pollution and gene-specific methylation in the Normative Aging Study: association, effect modification, and mediation analysis. Epigenetics. 2014; 9(3): 448-58.
111. Baccarelli A, Cassano PA, Litonjua A, et al. Cardiac autonomic dysfunction: effects from particulate air pollution and protection by dietary methyl nutrients and metabolic polymorphisms. Circulation. 2008; 117(14): 1802-9.
112. Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003; 22(1): 18-35.
113. Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: a review. Eur Respir J. 2008; 31(1): 179-97.
114. van der Vliet A, O’Neill CA, Cross CE, et al. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol. 1999; 276(2 Pt 1): L289-96.
115. Larsson N, Rankin GD, Bicer EM, et al. Identification of vitamin C transporters in the human airways: a cross-sectional in vivo study. BMJ Open. 2015; 5(4): e006979.
116. Niki E. Interaction of ascorbate and alpha-tocopherol. Ann N Y Acad Sci. 1987; 498: 186-99.
117. d’Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2003; 92(1): 88-95.
118. Siow RC, Richards JP, Pedley KC, Leake DS, Mann GE. Vitamin C protects human vascular smooth muscle cells against apoptosis induced by moderately oxidized LDL containing high levels of lipid hydroperoxides. Arterioscler Thromb Vasc Biol. 1999; 19(10): 2387-94.
119. Weber C, Erl W, Weber K, Weber PC. Increased adhesiveness of isolated monocytes to endothelium is prevented by vitamin C intake in smokers. Circulation. 1996; 93(8): 1488-92.
120. Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007; 43(1): 4-15.
121. Pacht ER, Kaseki H, Mohammed JR, Cornwell DG, Davis WB. Deficiency of vitamin E in the alveolar fluid of cigarette smokers. Influence on alveolar macrophage cytotoxicity. J Clin Invest. 1986; 77(3): 789-96.
122. Meydani M. Vitamin E and atherosclerosis: beyond prevention of LDL oxidation. J Nutr. 2001; 131(2): 366S-8S.
123. Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005; 25: 151-74.
124. Surette ME. The science behind dietary omega-3 fatty acids. CMAJ. 2008; 178(2): 177-80.
125. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006; 83(6 Suppl): 1505S-1519S.
126. Snijdewint FG, Kaliński P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993; 150(12): 5321-9.
127. Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991; 146(1): 108-13.
128. Fedyk ER, Phipps RP. Prostaglandin E2 receptors of the EP2 and EP4 subtypes regulate activation and differentiation of mouse B lymphocytes to IgE-secreting cells. Proc Natl Acad Sci USA. 1996; 93(20): 10978-83.
129. Sharma JN, Mohammed LA. The role of leukotrienes in the pathophysiology of inflammatory disorders: is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology. 2006; 14(1-2): 10-6.
130. Hallstrand TS, Henderson WR Jr. An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol. 2010; 10(1): 60-6.
131. Møller S, Lauridsen C. Dietary fatty acid composition rather than vitamin E supplementation influence ex vivo cytokine and eicosanoid response of porcine alveolar macrophages. Cytokine. 2006; 35(1-2): 6-12.
132. Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol. 2016; 785: 144-155.
133. Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim Biophys Acta. 2010; 1801(12): 1260-73.
134. Weber C, Erl W, Pietsch A, Danesch U, Weber PC. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 1995; 15(5): 622-8.
135. De Caterina R, Massaro M. Omega-3 fatty acids and the regulation of expression of endothelial pro-atherogenic and pro-inflammatory genes. J Membr Biol. 2005; 206(2): 103-16.
136. McVeigh GE, Brennan GM, Johnston GD, et al. Dietary fish oil augments nitric oxide production or release in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993; 36(1): 33-8.
137. Muto E, Hayashi T, Yamada K, Esaki T, Sagai M, Iguchi A. Endothelial-constitutive nitric oxide synthase exists in airways and diesel exhaust particles inhibit the effect of nitric oxide. Life Sci. 1996; 59(18): 1563-70.
138. Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002; 105(16): 1897-903.
139. Tong H, Rappold AG, Diaz-Sanchez D, et al. Omega-3 fatty acid supplementation appears to attenuate particulate air pollution-induced cardiac effects and lipid changes in healthy middle-aged adults. Environ Health Perspect. 2012; 120(7): 952-7.
140. Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009; 54(7): 585-94.
141. Harris WS, Kris-Etherton PM, Harris KA. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr Atheroscler Rep. 2008; 10(6): 503-9.
142. Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005; 112(13): 1945-52.
143. Xiao YF, Sigg DC, Leaf A. The antiarrhythmic effect of n-3 polyunsaturated fatty acids: modulation of cardiac ion channels as a potential mechanism. J Membr Biol. 2005; 206(2):141-54.
144. Leaf A. The electrophysiologic basis for the antiarrhythmic and anticonvulsant effects of n-3 polyunsaturated fatty acids: heart and brain. Lipids. 2001; 36 Suppl: S107-10.
145. Neki NS, Singh RB, Rastogi SS. How brain influences neuro-cardiovascular dysfunction. J Assoc Physicians India. 2004; 52: 223-30.
146. Christensen JH, Christensen MS, Dyerberg J, Schmidt EB. Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n-3 fatty acids. Am J Clin Nutr. 1999; 70(3): 331-7.
147. Kris-Etheron PM, Harris WS, Appel LJ. Omega-3 fatty acids and cardiovascular disease. New recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003; 23: 151-152.
148. Romieu I, Téllez-Rojo MM, Lazo M, et al. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med. 2005; 172(12): 1534-40.
149. Romieu I, Garcia-Esteban R, Sunyer J, et al. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM(2.5). Environ Health Perspect. 2008; 116(9): 1237-42.
150. Houghton CA, Fassett RG, Coombes JS. Sulforaphane and other nutrigenomic Nrf2 activators: Can the clinician’s expectation be matched by the reality? Oxid Med Cell Longev. 2016; 2016: 7857186.
151. Matusheski NV, Jeffery EH. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J Agric Food Chem. 2001; 49(12): 5743-9.
152. Wang GC, Farnham M, Jeffery EH. Impact of thermal processing on sulforaphane yield from broccoli (Brassica oleracea L. ssp. italica). J Agric Food Chem. 2012; 60(27): 6743-8.
153. Matusheski NV, Juvik JA, Jeffery EH. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry. 2004; 65(9): 1273-81.
154. Xu Z, Wang S, Ji H, et al. Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Sci Rep. 2016; 6: 30252
155. Harvey CJ, Thimmulappa RK, Sethi S, et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med. 2011; 3(78): 78ra32.
156. Munday R, Mhawech-Fauceglia P, Munday CM, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008; 68(5): 1593-600.
157. Tarozzi A, Angeloni C, Malaguti M, Morroni F, Hrelia S, Hrelia P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid Med Cell Longev. 2013; 2013:
158. Zhu H, Jia Z, Strobl JS, Ehrich M, Misra HP, Li Y. Potent induction of total cellular and mitochondrial antioxidants and phase 2 enzymes by cruciferous sulforaphane in rat aortic smooth muscle cells: cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol. 2008; 8(3): 115-25.
159. Keum YS. Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: implications of posttranslational modifications. Ann N Y Acad Sci. 2011; 1229:184-9.
160. Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002; 62(18): 5196-203.
161. Stefanson AL, Bakovic M. Dietary regulation of Keap1/Nrf2/ARE pathway: focus on plant-derived compounds and trace minerals. Nutrients. 2014; 6(9): 3777-801.
162. Boldogh I, Bacsi A, Choudhury BK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005; 115(8): 2169-79.
163. Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol. 2005; 115(2): 221-8; quiz 229.
164. Riedl MA, Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol. 2008; 8(1): 49-56.
165. Delfino RJ, Staimer N, Vaziri ND. Air pollution and circulating biomarkers of oxidative stress. Air Qual Atmos Health. 2011; 4(1): 37-52.
166. Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009; 130(3): 244-51.
167. Heber D, Li Z, Garcia-Lloret M, et al. Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food Funct. 2014; 5(1): 35-41.
168. Egner PA, Chen JG, Zarth AT, et al. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila). 2014; 7(8): 813-823.
169. Mohsenin V. Effect of vitamin C on NO2-induced airway hyperresponsiveness in normal subjects. A randomized double-blind experiment. Am Rev Respir Dis. 1987; 136(6): 1408-11.
170. Romieu I, Sienra-Monge JJ, Ramírez-Aguilar M, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002; 166(5): 703-9.
171. Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, et al. Antioxidant supplementation and nasal inflammatory responses among young asthmatics exposed to high levels of ozone. Clin Exp Immunol. 2004; 138(2): 317-22.
172. Trenga CA, Koenig JQ, Williams PV. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Arch Environ Health. 2001; 56(3): 242-9.
173. Samet JM, Hatch GE, Horstman D, et al. Effect of antioxidant supplementation on ozone-induced lung injury in human subjects. Am J Respir Crit Care Med. 2001; 164(5): 819-25.
174. Grievink L, Zijlstra AG, Ke X, Brunekreef B. Double-blind intervention trial on modulation of ozone effects on pulmonary function by antioxidant supplements. Am J Epidemiol. 1999; 149(4): 306-14.
175. Romieu I, Meneses F, Ramirez M, et al. Antioxidant supplementation and respiratory functions among workers exposed to high levels of ozone. Am J Respir Crit Care Med. 1998; 158(1): 226-32
176. Possamai FP, Júnior SÁ, Parisotto EB, et al. Antioxidant intervention compensates oxidative stress in blood of subjects exposed to emissions from a coal electric-power plant in South Brazil. Environ Toxicol Pharmacol. 2010; 30(2): 175-80.

