Micronutrients in mitigating the adverse health effects of air pollution
Part I: Health impacts and cellular mechanisms associated with exposure to polluted air
Abstract
Air pollution is a major environmental risk to human health and wellbeing. According to WHO reports in 2012, ambient (outdoor) and indoor air pollution was linked to seven million premature deaths worldwide. Most of them were attributed to cardiovascular diseases (stroke and ischemic heart disease), chronic obstructive pulmonary disease (COPD), lung cancer, and acute lower respiratory infections in children. Yet 92% of the world population lives in places that exceed the WHO air quality guidelines. On the other hand, laboratory and clinical studies indicate that a nutritious diet and/or intake of micronutrients with antioxidant, anti-inflammatory, and detoxifying properties may ameliorate many harmful health effects caused by polluted air.
This review is composed of two parts: Part I addresses cellular and health aspects associated with human exposure to polluted air and Part II (to be published in the next edition of our Journal) will discuss potential biological mechanisms underlying the protective effects of vitamins B, C, and E, omega-3 fatty acids, and sulforaphane against air pollutants, as demonstrated in human studies.
Characteristics of air pollution
Air pollution comes from many sources and encompasses diverse dispersed mixtures of gaseous, liquid, and solid compounds that adversely affect humans and ecosystems.
Natural sources: Some air pollutants come from natural sources, such as radon gas from radioactive decay, smoke and carbon monoxide from wildfires, or sulfur and ash particles from volcanic activity.
Indoor sources: Among many other sources cooking and heating homes with fireplaces or traditional stoves using solid fuels creates high levels of indoor air pollution, exposing occupants to fine particles, carbon monoxide, and other health damaging pollutants4Burden of disease from Household Air Pollution for 2012. World Health Organization. www.int/phe/health_topics/outdoorair/databases/FINAL_HAP_AAP_BoD_24March2 014.pdf.
In addition, larger particles of dust, pollen, spores and plant and insect parts suspended in air contribute to pollution. Also many modern materials (e.g. construction materials), office equipment (e.g. printers), cleaning products, or indoor activities such as smoking or burning candles emit hazardous substances (e.g., benzene, formaldehyde, naphthalene, ozone, phthalates)13Franken C, Lambrechts N, Govarts E, et al. Phthalate-induced oxidative stress and association with asthma-related airway inflammation in adolescents. Int J Hyg Environ Health. 2017; 220: 468-477., 14Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC.Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco and menthol-flavored e-cigarettes. Environ Health. 2017; 16(1): 42., 15WHO guidelines for indoor air quality: selected pollutants. Web site. http://www.euro.who.int/data/assets/pdf_file/0009/128169/e94535.pdf.
Industrial sources: Industrial pollution, largely linked to burning multiple types of fuel including coal, charcoal, wood, crop waste, dung, gas, naphtha, and many others, has the most pronounced negative effects on health 1Arbex MA, Santos Ude P, Martins LC, Saldiva PH, Pereira LA, Braga AL. Air pollution and the respiratory system. J Bras Pneumol. 2012; 38: 643-655., 2Bernstein JA, Alexis N, Barnes C et al. Health effects of air pollution. J Allergy Clin Immunol. 2004; 114:1116-1123., 3Burden of disease from Ambient Air Pollution for 2012. World Health Organization. www.int/phe/health_topics/outdoorair/databases/AAP_BoD_results_March2014.pdf, 4Burden of disease from Household Air Pollution for 2012. World Health Organization. www.int/phe/health_topics/outdoorair/databases/FINAL_HAP_AAP_BoD_24March2 014.pdf, 5Household Air Pollution fact sheet N292.World Health Organization. Web site. www.who.int/mediacentre/factsheets/fs292/en/, 6Prennise D. Biomass Pollution Basics. Center for Entre- preneurship in International Health and Development (CEIHD). Web site. who.int/indoorair/interventions/antiguamod21.pdf.
Pollutants are classified as primary – directly emitted from the source – or secondary, which are formed in the air as a result of further chemical and photochemical reactions. Chemical composition and concentrations of pollutants in the air vary, depending on sources and magnitude of emissions as well as atmospheric conditions, terrain, and other local and even global factors.
The six common air pollutants known as “criteria air pollutants” regulated by the U.S. Environmental Protection Agency and other governmental institutions around the globe include sulfur dioxide (SO2), nitrogen dioxide (NO2), carbon monoxide (CO), ozone (O3), lead (Pb), and particulates alternatively referred to as “particulate matter” (PM)7NAAQS Table.United States Environmental Protection Agency. Web site. www.epa.gov/criteria-air-pollutants/naaqs-table.
PM encompasses all solid and liquid particles suspended in air, many of which are hazardous. PM may include a myriad of compounds of different origin including a variety of ions (e.g., SO42-, NO3-, NH4+), toxic metals (e.g., Pb, Al, Hg, As), volatile organic compounds (VOC), polycyclic aromatic hydrocarbons (PAHs) such as benzo(a)pyrene, and various allergens. Particulates with an aerodynamic diameter of 10 μm or less (PM10), 2.5 μm or less (PM2.5; fine particles), and 0.1 μm or less (PM0.1) described as ultrafine particles (UFPs), have the strongest impact on human health, due to their ability to reach the air ducts in the lungs and pulmonary tree and even penetrate into the systemic circulation 8Lee BJ, Kim B, Lee K. Air pollution exposure and cardiovascular disease. Toxicol Res. 2014; 30: 71-75., 9Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011; Article ID 487074., 10Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008; 122: 456-468..
In particular, inhaled UFPs are efficiently deposited in nasal, tracheobronchial, and alveolar regions from where they can translocate to other extra-pulmonary organs including liver, kidneys, heart and brain 11Kim JW, Park S, Lim CW, Lee K, Kim B. The role of air pollutants in initiating liver disease. Toxicol Res. 2014; 30(2): 65–70., 12Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004; 16(6-7): 437-45..
UFPs are ubiquitous in urban air both indoors and outdoors.
Chemical characteristic of the most common pollutants
Many pollutants in addition to their own intrinsic chemical toxicity, share a common property of being potent oxidants [9,10]. Atmospheric gases namely O3, NO2, SO2, CO, as well as compounds carried by particulates of all sizes, are known to form reactive oxygen species (ROS) such as superoxide anion, hydrogen peroxide, and hydroxyl radicals, or indirectly activate intracellular oxidant pathways 9Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011; Article ID 487074., 10Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008; 122: 456-468., 16Brook RD, Rajagopalan S, Pope CA 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010; 121(21): 2331-78.[/tooltip], 17.
Regarding particulates (PM), the size and chemical composition determine their biological effects. Many studies show that the smaller their size the higher the redox activity, alveolar deposition and potential to elicit oxidative and inflammatory injury 18Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008; 26(4): 339-62., 19Jeng HA. Chemical composition of ambient particulate matter and redox activity. Environ Monit Assess.2010; 169(1-4): 597-606..
Chemical analysis of PM from urban areas with heavy vehicle traffic revealed that ultrafine particles (UFPs) have higher concentrations of pro-oxidants such as PAHs, organic carbon, and elemental carbon than PM2.5 19Jeng HA. Chemical composition of ambient particulate matter and redox activity. Environ Monit Assess.2010; 169(1-4): 597-606.. On the contrary, the content of transition metals that may generate ROS by Fenton‘s reaction was higher in fine and coarse particles (PM10-2.5) than in UFPs 20Li N, Sioutas C, Cho A. et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003; 111(4): 455-60..
Overall, studies suggest that many outdoor and indoor pollutants cause oxidative damage to proteins, lipids, and DNA, and trigger pro-inflammatory responses resulting in multiple pathologies 9Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011; Article ID 487074., 10Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008; 122: 456-468..
In this regard, Li et al. proposed a hierarchical (three- tiered) model of cellular responses to incremental levels of oxidative stress using diesel exhaust particles (DEPs), as an exemplary pollutant (Fig. 1) 21Li N, Hao M, Phalen RF, Hinds WC, Nel AE. et al. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. ClinImmunol. 2003; 109(3): 250-65..
According to this, a low level of oxidative stress (tier 1) initiates cyto-protective mechanisms through up-regulation of antioxidant and detoxification enzymes (phase II drug metabolism), such as HO-1, GST, NQO1. However, if these protective mechanisms fail, higher intensity of oxidative stress triggers more damaging pro-inflammatory responses via activation of mitogen- activated protein kinase (MAPK) and nuclear factor kappa B (NFkB) cascade (tier 2). At the highest level of oxidative stress (tier 3), perturbation of the mitochondrial permeability and disruption of electron transfer occurs, resulting in cellular apoptosis or toxic necrosis 21Li N, Hao M, Phalen RF, Hinds WC, Nel AE. et al. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. ClinImmunol. 2003; 109(3): 250-65..
Taken together, the hierarchical oxidative stress model predicts that weakened antioxidant and detoxification mechanisms could be responsible for aggravation of the pathological processes, simultaneously indicating the importance of antioxidant support as the first line of antioxidant defense.
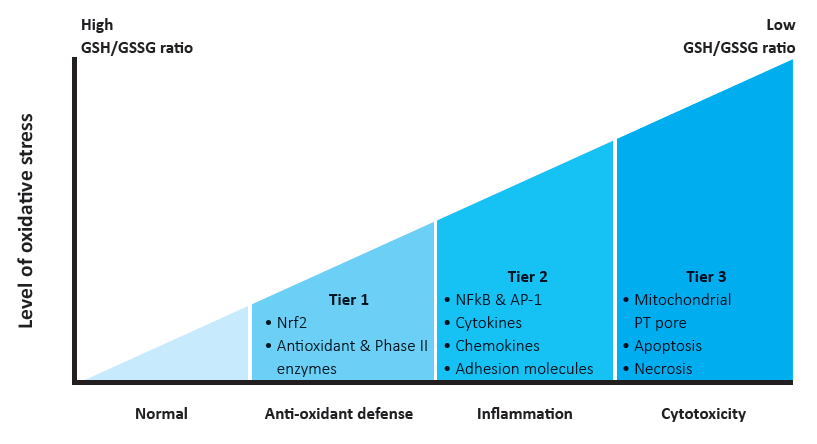
Figure 1 – Hierarchical oxidative stress model. GSH/GSSG ratio, the ratio of reduced glutathione (GSH) to oxidized GSH (GSSG) [22, modified].
2. Health consequences of exposure to polluted air
Epidemiological and clinical studies conducted in Europe, US, China and many countries across the world consistently show a vast array of adverse health effects related to both short- and long-term exposure to air pollution [10,23,24,25,26,27]. The link between certain air pollutants and an increase in general morbidity and mortality is well established. The most frequent causes of hospital admission and/or death associated with exposure to pollutants are respiratory problems (e.g., acute lung respiratory infection, COPD, asthma and lung cancer) and cardiovascular problems (e.g., stroke, ischemic heart disease, arrhythmia) [3,4,8,10,16,23,25,28]. The susceptibility to specific pollutants and severity of symptoms may vary depending on age, gender, genetic background, pre- existing diseases, and nutrition [10,16].
2.1 Impact of air pollution on the respiratory system
Upper and lower respiratory tracts are the first target for air pollution and the first line of defense against it. Inhaled pollutants trigger diverse unfavorable reactions, which may contribute to the pathogenesis and/or exacerbation of respiratory diseases [10]. Rhinorrhea, sneezing, nasal obstruction, coughing, laryngospasm, dyspnea, wheezing, hyper-responsiveness to allergens, and frequent pulmonary infections constitute a list of common respiratory-related symptoms experienced after and with exposure to high levels of air pollutants [1]. Often these symptoms are accompanied by impaired pulmonary function, such as reversible reductions in forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and forced expiratory flow between 25-75% of the FVC (FEF25-75) [29,30,31]. In turn, chronic exposure to pollutants has been associated with higher incidents of asthma, COPD, pneumonia, and lung cancer as well as chronic decrements in aforementioned spirometric parameters (FVC, FEV1, FEF25-75) [1].
Age-related susceptibility to air pollution: The most vulnerable to developing life-threatening and/or chronic complications are children, the elderly, individuals carrying specific gene variants, and patients with pre-existing diseases like asthma, COPD, cystic fibrosis, diabetes, as well as those suffering from various cardiovascular conditions.
The high susceptibility of children results mainly from their not yet fully-developed cellular defense mechanisms, higher volume of air inhaled per body weight compared to adults, and importantly, still immature lung tissue [32,33]. Concerning the latter, the respiratory system begins its development at the fourth week of pregnancy and at the end of the seventh week the lungs are already developed. However, lung maturation continues after birth and 80% of the alveolar tissue is fully developed by the end of adolescence [32]. Consequently, lungs are susceptible to potential damage and impairment in their development, which can underlie the onset of pulmonary disease later in life.
As for the elderly, accumulated evidence suggests that their increased susceptibility to the adverse effects of air pollutants is related to age-associated decline in lung function, underlying pulmonary and/or cardiovascular conditions, and reduction in antioxidant defense capacity of the fluid lining the respiratory tract (RTLF) [10]. This fluid contains a range of low molecular weight antioxidants (glutathione, ascorbic acid, tocopherol, uric acid), antioxidant enzymes (glutathione peroxidase, superoxide dismutase, catalase), and the metal-binding proteins (ceruloplasmin, transferrin) all working together as a robust antioxidant defense system [34]. Therefore, proper composition and quantity of antioxidants in RTLF is critical for optimal lung function.
Regardless of age genetic background, in particular polymorphism in TNF-alpha, GSTM1, GSTP1, NQO1, TLR4, and NRF2 genes, plays a substantial role in pulmonary responses to air pollution [35,36,37,38,39]. These genes encode proteins involved in the management of oxidative stress and inflammation.
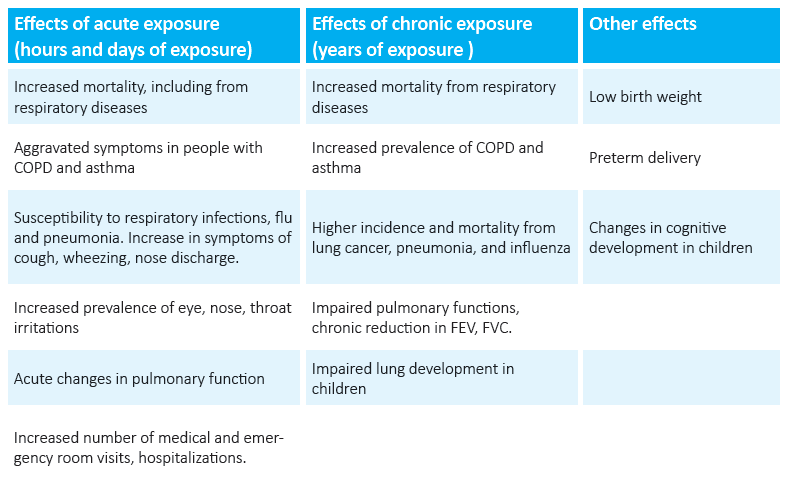
Table 1: Acute and chronic effects of exposure to pollutants on the respiratory system
Cellular mechanisms associated with lung exposure to air pollution:
The core processes underlying both acute and chronic exposure of the lung airway system to pollutants include oxidative stress and inflammation.
Due to their pro-oxidant character, most pollutants directly or indirectly elicit oxidative stress, which if not attenuated, triggers pro-inflammatory responses that further potentiate ROS generation [10]. This is evidenced by higher levels of oxidative and inflamm- atory markers measured in breath condensates, sputum, broncho-alveoral lavage fluid, blood and urine in people with asthma, COPD, acute respirato- ry distress syndrome, and cystic fibrosis, as well as in healthy children and adults exposed to air pollution [10,32,40,41,42,43,44].
Lipids: A primary consequence of oxidative stress is oxidative degeneration of lipids, mainly polyunsaturated fatty acids (PUFAs) in cell membranes, which can lead to permanent cell membrane dysfunction, damage and ultimately cell death. Moreover, the end products of lipid peroxidation such as 4-hydroxynonenal (HNE) or lipid ozonation products (LOPs) prompt numerous downstream effects. For instance LOPs can activate phospholipases A2, C and D and increase a release of pro-inflammatory mediators by bronchial epithelial cells such as platelet-activating factor, prostaglandin E2 (PGE2), interleukins (IL-6 and IL-8), whereas HNE contributes to airway remodeling by activating epidermal growth factor receptor and inducing fibronectin production in lung fibroblasts [45,46,47].
Proteins: Free radicals may alter the structure and function of proteins by oxidation of the polypeptide backbone, causing protein aggregation by formation of protein-protein cross-links, inducing peptide bond cleavage, or amino acid side chain modifications [10]. It has been demonstrated that oxidation of methionine residues in alpha(1)-antitrypsin, the primary inhibitor of neutrophil elastase, exposes the alveolar surface to destruction by neutrophil elastase, which eventually may lead to emphysema [48]. Another example of proteins vulnerable to oxidative damage are surfactant proteins (SPs). Oxidation of SP-B and SP-C can abrogate the ability of the surfactant film to reduce lung surface tension during breathing, while oxidative changes in SP-A and SP-D impair their capacity to agglutinate bacteria and enhance phagocytosis by macrophages [49,50,51,52].
DNA: Free radicals are also involved in damaging the DNA with consequences such as alternations in gene expression, triggering cell death, and the development of cancer [53,54]. A study from Mexico revealed a high systemic DNA damage among outdoor workers exposed to high levels of PM2.5, ozone, and VOC [55]. This is consistent with laboratory experiments showing an increase in biomarkers of oxidative DNA damage formation (8-OHdG and 8-oxo-dG) in human airway epithelial cells after PM and diesel exhaust particle (DEP) exposure [56,57]. It is suggested that the metal content of particulates plays a key role in this process. Indeed, certain metals, notably chromium, nickel, cobalt, iron, and copper were found to induce oxidative DNA damage in the presence of hydrogen peroxide or various organic carcinogens [58]. DNA breaks and oxidative DNA damage in lung epithelial cells were also observed after ozone exposure [59]. Another study revealed that ozone, by generating hydroxyl radicals, caused DNA backbone cleavage in addition to DNA base modifications, which were mainly ascribed to its direct effects [60].
Neuronal functions: It is worth noting that ground- level ozone has been strongly associated with impaired lung function, in both healthy people and asthmatics. It can directly and indirectly, via inflammatory molecules (e.g. PGE2), stimulate nociceptive bronchopulmonary nerves, namely airway C-fibers, thereby inducing bronchoconstriction reflex [61,62,63]. In addition, C-fiber stimulation releases neuropeptides such as substance P, which is involved in contraction of smooth muscle in airways, activation of mucous glands, and dilation of nearby capillaries [64,65]. These actions are also enhanced by airway mucosal inflammation, for instance by autocoids such as PGE2, which is released from bronchial epithelial cells upon ozone exposure. PGE2, together with other inflammatory mediators, can attract polymorphonuclear leukocytes into the lung, activate alveolar macrophages, and initiate a cascade of events leading to lung inflammation [63,64,66,67,68].
In summary, the above examples just scratch the surface of the complexity and deleterious effects of free radicals which are not limited to ROS however, but also include reactive nitrogen species (RNS), reactive sulfur species (RSS), and reactive chlorine species (RCS) [53]. Furthermore, aside from exogenous sources of free radicals such as air pollution, tobacco smoke etc., cellular physiological processes (enzymatic and non- enzymatic) involving mitochondria, cytochrome P450, and peroxisomes as well as activated immune cells, represent a significant portion of the oxidative stress burden [53].
2.2. Effects of polluted air on cardiovascular functions
Short- and long-term exposure to air pollution has been associated with the development and aggravation of ischemic heart disease, heart failure, cardiac arrest, cardiac arrhythmia, ischemic stroke, transient ischemic attack, atherosclerosis, and peripheral arterial and venous diseases, especially deep vein thrombosis [16,69].
The main mechanisms implicated in these effects include systemic inflammation, systemic oxidative stress, increased thrombosis and coagulation, vascular dysfunction, decreased plaque stability, and autonomic nervous system imbalance, triggering arrhythmias [16,70]. This is corroborated by human and animal studies demonstrating the effects of air pollution on inflammation markers (e.g., CRP, IL-6, TNF-alpha), oxidative stress (e.g., 8-iso-prostaglandin F2alpha), coagulation (e.g., fibrinogen), and endothelial dysfunction (e.g., ICAM-1, VCAM-1, ET-1) [16,70].
A study by Bind et al. demonstrated that increases in particle number (PN), black carbon (BC), NO2 and CO were associated with elevated levels of fibrinogen in plasma, while ozone was a significant predictor of CRP and ICAM-1 in elderly men [70]. Adhesion molecules, ICAM-1 and VCAM-1 also have been correlated with PN, BC, NO2, CO, PM2.5, and SO42-. Interestingly, the authors revealed that individual responses to air pollutants vary depending on person‘s baseline DNA methylation status. Moreover, recent human intervention studies provided strong evidence that ambient PM2.5 could induce rapid decrease in DNA methylation in pro-inflammatory (CD40LG), pro- coagulant (F3, SERPINE1) and pro-vasoconstriction (ACE, EDN1) genes, thereby enhancing their expression [71,72].
In another study, a two-hour inhalation of environmentally relevant concentrations of ambient PM2.5 (150 μg/m3) plus ozone (120 ppb) caused acute arterial vasoconstriction in healthy humans [73]. The proposed mechanisms include spontaneous increase in sympathetic nervous system activity via stimulation of pulmonary vagal afferents or an oxidative stress- induced increase in vascular endothelin release, analogous to the effects of cigarette smoking. In addition, in a similar exposure to PM2.5 and ozone there was observed a significant increase in diastolic blood pressure (by 9.3%) which was associated with the PM2.5 organic carbon content [74]. Again, the plausible mechanisms pointed toward the activation of lung macrophages or alveolar cells by pollutants with the subsequent promotion of systemic oxidative stress and pro-inflammatory responses, or an increase in sympathetic drive. It is suggested that PM-stimulated lung macrophages, via cytokine signaling, can promote the release of immature leukocytes from bone marrow, elevating the pool of circulating leukocytes and facilitating leukocyte-mediated inflammation – a hallmark of cardiovascular disease [75,76,77,78].
A study by Schwartz el al. corroborate that ROS and inflammation play a key role in PM-induced cardiac autonomic dysfunction [79]. The authors studied the effect of glutathione S-transferase gene (GSTM1) deletion, common in approximately half of the white population, on PM-associated reductions in heart rate variability (HRV). It is known that parasympathetic and sympathetic stimulation of the heart affects intervals between normal heart beats and that reduced HRV is a strong predictor of sudden cardiac death. The study showed that about 10 μg/m3 increase in the exposure to PM2.5, decreased the HRV by 34% in subjects without GSTM1 gene, however it did not affect those with GSTM1 present. Furthermore, individuals with obesity or higher neutrophil counts (above the average baseline systemic inflammation and oxidative stress) even with the GSTM1 gene were more affected by PM exposure. At the same time, a combination of GSTM1- null genotype and obesity or high neutrophil counts resulted in over 50% reductions in HRV, thereby an increased risk of sudden cardiac death [79].
It has been shown that air pollution also affects the delicate dynamic balance between blood clot formation and blood clot dissolution [80]. In human controlled- exposure studies, inhalation of diluted diesel exhaust resulted in markedly increased thrombus formation, platelet-neutrophil and platelet-monocyte aggregates in addition to the impairment of vascular function, tissue plasminogen activator (t-PA) release, and fibrinolysis [81,82]. Data from the study by Kilinc et al. imply that particulates, including UFPs, promote early pro-coagulant actions, mostly through the tissue factor-driven (extrinsic) coagulation pathway, whereas long-lasting thrombogenic effects are predominantly mediated by formation of activated factor XII [83]. Overall, a collective body of evidence indicates that air pollution impacts all vital aspects of cardiovascular system function via three hypothetically-defined pathways [16].
Pathway 1 (sub-acute and chronic effects)
Encompasses biological trails of systemic spill-over of pulmonary oxidative stress and inflammation (cytokines, activated immune cells, platelets, possibly histamine, endothelin, oxidized lipids).
Pathway 2 (acute effects)
Refers to pollutant interactions with lung receptors or nerves affecting the systemic equilibrium of the autonomic nervous system (e.g., vasoconstriction, increased platelet aggregation) or heart rhythm.
Pathway 3 (acute and possibly chronic effects)
Pertains to direct effects of the translocation of air pollutants, in particular UFPs or particle constituents (metals, organic compounds), into the systemic circulation, causing oxidative stress, inflammation, endothelial dysfunction, atherosclerosis, vasoconstriction and other effects.
References
1. Arbex MA, Santos Ude P, Martins LC, Saldiva PH, Pereira LA, Braga AL. Air pollution and the respiratory system. J Bras Pneumol. 2012; 38: 643-655.
2. Bernstein JA, Alexis N, Barnes C et al. Health effects of air pollution. J Allergy Clin Immunol. 2004; 114:1116-1123.
3. Burden of disease from Ambient Air Pollution for 2012. World Health Organization. www.int/phe/health_topics/outdoorair/databases/AAP_BoD_results_March2014.pdf
4. Burden of disease from Household Air Pollution for 2012. World Health Organization. www.int/phe/health_topics/outdoorair/databases/FINAL_HAP_AAP_BoD_24March2 014.pdf
5. Household Air Pollution fact sheet N292.World Health Organization. Web site. www.who.int/mediacentre/factsheets/fs292/en/
6. Prennise D. Biomass Pollution Basics. Center for Entre- preneurship in International Health and Development (CEIHD). Web site. who.int/indoorair/interventions/antiguamod21.pdf
7. NAAQS Table.United States Environmental Protection Agency. Web site. www.epa.gov/criteria-air-pollutants/naaqs-table
8. Lee BJ, Kim B, Lee K. Air pollution exposure and cardiovascular disease. Toxicol Res. 2014; 30: 71-75.
9. Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011; Article ID 487074.
10. Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008; 122: 456-468.
11. Kim JW, Park S, Lim CW, Lee K, Kim B. The role of air pollutants in initiating liver disease. Toxicol Res. 2014; 30(2): 65–70.
12. Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004; 16(6-7): 437-45.
13. Franken C, Lambrechts N, Govarts E, et al. Phthalate-induced oxidative stress and association with asthma-related airway inflammation in adolescents. Int J Hyg Environ Health. 2017; 220: 468-477.
14. Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC.Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health. 2017; 16(1): 42.
15. WHO guidelines for indoor air quality: selected pollutants. Web site. www.euro.who.int/ data/assets/pdf_file/0009/128169/e94535.pdf
16. Brook RD, Rajagopalan S, Pope CA 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010; 121(21): 2331-78.
17. Piantadosi CA. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic Biol Med. 2008; 45(5): 562-9.
18. Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008; 26(4): 339-62.
19. Jeng HA. Chemical composition of ambient particulate matter and redox activity. Environ Monit Assess.2010; 169(1-4): 597-606.
20. Li N, Sioutas C, Cho A. et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003; 111(4): 455-60.
21. Li N, Hao M, Phalen RF, Hinds WC, Nel AE. et al. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. ClinImmunol. 2003; 109(3): 250-65.
22. Meng H, Xia T, George S , Nel AE. A predictive toxicological paradigm for the safety assessment of nanomaterials. ASC Nano. 2009; 3(7): 1620-7.
23. Kloog I, Coull BA, Zanobetti A, Koutrakis P, Schwartz JD. Acute and chronic effects of particles on hospital admissions in New-England. Plos One. 2012; 7(4): e34664.
24. Berglund D. Long-Term Effects of Air Pollution. WJM. 1996; 165(3): 140. www.ncbi.nlm.nih.gov/pmc/articles/PMC1303722/pdf/westjmed003480042a.pdf
25. Vigotti MA. Short-term effects of exposure to urban air pollution on human health in Europe. The APHEA Projects (Air Pollution and Health: a European Approach). Epidemiol Prev. 1999; 23(4): 408-15.
26. Lü J, Liang L, Feng Y, Li R, Liu Y. Air Pollution Exposure and Physical Activity in China: Current Knowledge, Public Health Implications, and Future Research Needs. Int J Environ Res Public Health. 2015; 12(11): 14887-97.
27. Péter S, Holguin F, Wood LG. et al. Nutritional Solutions to Reduce Risks of Negative Health Impacts of Air Pollution. Nutrients. 2015; 7(12): 10398-416.
28. Brook RD. Cardiovascular effects of air pollution. ClinSci (Lond). 2008; 115(6): 175-87.
29. Liu L, Poon R, Chen L, et al. Acute effects of air pol- lution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009; 117(4): 668-74.
30. Chang YK, Wu CC, Lee LT, Lin RS, Yu YH, Chen YC. The short-term effects of air pollution on adolescent lung function in Taiwan. Chemosphere. 2012; 87(1): 26-30.
31. McCreanor J, Cullinan P, Nieuwenhuijsen MJ. et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007; 357(23): 2348-58.
32. Fortoul TI, Rojas-Lemus M, Rodriguez-Lara V et al. Air pollution and its effects in the respiratory system. In: The Impact of Air Pollution on Health, Economy, Environment and Agricultural Sources, Dr. Mohamed Khallaf (Ed.), InTech, 2011. DOI: 10.5772/17766.
33. Salvi S. Health effects of ambient air pollution in children. Paediatr Respir Rev. 2007; 8(4): 275-80.
34. Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003; 60(8): 612-6.
35. Chen C, Arjomandi M, Tager IB, Holland N, Balmes JR. Effects of antioxidant enzyme polymorphisms on ozone-induced lung function changes. Eur Respir J. 2007; 30(4): 677-83.
36. Fagerås Böttcher M, Hmani-Aifa M, Lindström A. et al. A TLR4 polymorphism is associated with asthma and reduced lipopolysaccharide-induced interleukin-12(p70) responses in Swedish children. J Allergy ClinImmunol. 2004; 114(3): 561-7.
37. Li YF, Gauderman WJ, Avol E, Dubeau L, Gilliland FD. Associations of tumor necrosis factor G-308A with childhood asthma and wheezing. Am J Respir Crit Care Med. 2006; 173(9): 970-6.
38. David GL, Romieu I, Sienra-Monge JJ et al. Nico- tinamide adenine dinucleotide (phosphate) reduced: quinone oxidoreductase and glutathione S-transferase M1 polymorphisms and childhood asthma. Am J Respir Crit Care Med . 2003; 168(10): 1199-204.
39. Masuko H, Sakamoto T, Kaneko Y. et al. An interaction between Nrf2 polymorphisms and smoking status affects annual decline in FEV1: a longitudinal retrospective cohort study. BMC Med Genet. 2011; 20; 12: 97.
40. De Prins S, Dons E, Van Poppel M. et al. Airway oxidative stress and inflammation markers in exhaled breath from children are linked with exposure to black carbon. Environ Int. 2014; 73: 440-6.
41. Rosa MJ, Yan B, Chillrud SN. et al. Domestic airborne black carbon levels and 8-isoprostane in exhaled breath condensate among children in New York City. Environ Res. 2014; 135: 105-10.
42. Patel MM, Chillrud SN, Deepti KC, Ross JM, Kinney PL. et al. Traffic-related air pollutants and exhaled markers of airway inflammation and oxidative stress in New York City adolescents. Environ Res. 2013; 121: 71-8.
43. Huang W, Wang G, Lu SE. et al. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med. 2012; 186(11): 1150-9.
44. Li W, Wilker EH, Dorans KS. et al. Short-Term Expo- sure to Air Pollution and Biomarkers of Oxidative Stress: The Framingham Heart Study. J Am Heart Assoc. 2016; 28, 5(5).
45. Tsukagoshi H, Kawata T, Shimizu Y, Ishizuka T, Dobashi K, Mori M. 4-Hydroxy-2-nonenal enhances fibronec- tin production by IMR-90 human lung fibroblasts partly via activation of epidermal growth factor receptor-linked extracellular signal-regulated kinase p44/42 pathway. Toxicol Appl Pharmacol. 2002; 184(3): 127-35.
46. Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Toxicol Appl Pharmacol. 1998; 150(2): 338-49.
47. Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Induction of inflammatory mediators in Lipid ozonation products activate phospholipases A2, C, and D. human airway epithelial cells by lipid ozonation products. Am J Respir Crit Care Med. 1999; 160(6): 1934-42.
48. Taggart C, Cervantes-Laurean D, Kim G. et al. Oxida- tion of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem. 2000; 275: 27258-27265.
49. Mikerov AN, Umstead TM, Gan X. et al. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol. 2008; 294(1): L121-30
50. Manzanares D, Rodriguez-Capote K, Liu S. et al. Mo- dification of tryptophan and methionine residues is implicated in the oxidative inactivation of surfactant protein B. Biochemistry. 2007;46(18): 5604-5615.
51. Rodríguez-Capote K, Manzanares D, Haines T, Poss- mayer F. Reactive oxygen species inactivation of surfactant involves structural and functional alterations to surfactant proteins SP-B and SP-C. Biophys J. 2006; 90(8): 2808-2821.
52. Starosta V, Griese M. Oxidative damage to surfactant protein D in pulmonary diseases. Free Radic Res. 2006; 40(4): 419-425.
53. Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013; 10(9): 3886-3907.
54. Møller P, Folkmann JK, Forchhammer L. et al. Air pollution, oxidative damage to DNA, and carcinogenesis. Cancer Lett. 2008; 266(1): 84-97.
55. Tovalin H, Valverde M, Morandi MT, Blanco S, White- head L, Rojas E. DNA damage in outdoor workers occupationally exposed to environmental air pollutants. Occup Environ Med. 2006; 63(4): 230-236.
56. Prahalad AK, Inmon J, Dailey LA, Madden MC, Ghio AJ, Gallagher JE. Air pollution particles mediated oxi- dative DNA base damage in a cell free system and in human airway epithelial cells in relation to particulate metal content and bioreactivity. Chem Res Toxicol. 2001; 14(7): 879-887.
57. Tokiwa H, Sera N, Nakanishi Y, Sagai M. 8-Hydroxy- guanosine formed in human lung tissues and the association with diesel exhaust particles. Free Radic Biol Med. 1999; 27(11-12): 1251-1258.
58. Kawanishi S, Hiraku Y, Murata M, Oikawa S. The role of metals in site-specific DNA damage with reference to carcinogenesis. Free Radic Biol Mec. 2002;32(9): 822-832.
59. Cheng TJ, Kao HP, Chan CC, Chang WP. Effects of ozone on DNA single-strand breaks and 8-oxoguanine formation in A549 cells. Environ Res. 2003; 93(3): 279-284.
60. Ito K, Inoue S, Hiraku Y, Kawanishi S. Mechanism of site-specific DNA damage induced by ozone. Mutat Res. 2005; 585(1-2): 60-70.
61. Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol. 2010; 588(Pt 3): 423-433.
62. Barker JS, Wu Z, Hunter DD, Dey RD. Ozone exposure initiates a sequential signaling cascade in airways involving interleukin-1beta release, nerve growth factor secretion, and substance P upregulation. J Toxicol Environ Health A. 2015; 78(6): 397-407.
63. Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001; 125(1-2): 47-65.
64. Health Effects of Ozone in the General Population. United States Environmental Protection Agency. Web site. www.epa.gov/ozone-pollution-and-your-patients-health/health-effects-ozone-general-population
65. Schelegle ES, Walby WF. Vagal afferents contribute to exacerbated airway responses following ozone and allergen challenge. Respir Physiol Neurobiol. 2012; 181(3): 277-285.
66. Lee LY, Widdicombe JG. Modulation of airway sensitivity to inhaled irritants: role of inflammatory mediators. Environ Health Perspect. 2001; 109 Suppl 4:585-589.
67. McKinnon KP, Madden MC, Noah TL, Devlin RB. In vitro ozone exposure increases release of arachidonic acid products from a human bronchial epithelial cell line. Toxicol Appl Pharmacol. 1993; 118(2): 215-223.
68. Leikauf GD, Simpson LG, Santrock J. et al. Airway epithelial cell responses to ozone injury. Environ Health Perspect. 1995; 103 Suppl 2: 91-95.
69. Du Y, Xu X, Chu M, Guo Y, Wang J. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis. 2016; 8(1): E8-E19.
70. Bind MA, Baccarelli A, Zanobetti A, et al. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology. 2012; 23(2): 332-340.
71. Chen R, Meng X, Zhao A. et al. DNA hypomethylation and its mediation in the effects of fine particulate air pollution on cardiovascular biomarkers: A randomized crossover trial. Environ In. 2016; 94: 614-619.
72. Wang C, Chen R, Cai J. et al. Personal exposure to fine particulate matter and blood pressure: A role of angiotensin converting enzyme and its DNA methylation. Environ Int. 2016; 94: 661-666.
73. Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002; 105, 1543-1536.
74. Urch B, Silverman F, Corey P. et al. Acute blood pres- sure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005; 113, 1052-1055.
75. Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am J Epidemiol. 2001; 154, 758-764.
76. Salvi S, Blomberg A, Rudell B. et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999; 159, 702-709.
77. Mukae H, Vincent R, Quinlan K. et al. The effect of repeated exposure to particulate air pollution (PM10) on the bone marrow. Am J Respir Crit Care Med. 2001; 163, 201-209.
78. Goto Y, Ishii H, Hogg JC. et al. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am J Respir Crit Care Med. 2004; 170, 891-897.
79. Schwartz J, Park SK, O‘Neill MS, et al. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005; 172, 1529-1533.
80. Franchini M, Guida A, Tufano A, Coppola A. Air pollution, vascular disease and thrombosis: linking clinical data and pathogenic mechanisms. J Thromb Haemost. 2012; 10, 2438-2451.
81. Lucking AJ, Lundback M, Mills NL, et al. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J. 2008; 24, 3043-3051.
82. Mills NL, Törnqvist H, Robinson SD, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Cirulation. 2005; 112, 3930-3936.
83. Kilinç E, Van Oerle R, Borissoff JI, et al. Factor XII acti- vation is essential to sustain the procoagulant effects of particulate matter. J Thromb Haemost. 2011; 9, 1359-139

Figure 1 – Hierarchical oxidative stress model. GSH/GSSG ratio, the ratio of reduced glutathione (GSH) to oxidized GSH (GSSG) [22, modified].
Table 1: Acute and chronic effects of exposure to pollutants on the respiratory system

References
1. Arbex MA, Santos Ude P, Martins LC, Saldiva PH, Pereira LA, Braga AL. Air pollution and the respiratory system. J Bras Pneumol. 2012; 38: 643-655.
2. Bernstein JA, Alexis N, Barnes C et al. Health effects of air pollution. J Allergy Clin Immunol. 2004; 114:1116-1123.
3. Burden of disease from Ambient Air Pollution for 2012. World Health Organization. www.int/phe/health_topics/outdoorair/databases/AAP_BoD_results_March2014.pdf
4. Burden of disease from Household Air Pollution for 2012. World Health Organization. www.int/phe/health_topics/outdoorair/databases/FINAL_HAP_AAP_BoD_24March2 014.pdf
5. Household Air Pollution fact sheet N292.World Health Organization. Web site. www.who.int/mediacentre/factsheets/fs292/en/
6. Prennise D. Biomass Pollution Basics. Center for Entre- preneurship in International Health and Development (CEIHD). Web site. who.int/indoorair/interventions/antiguamod21.pdf
7. NAAQS Table.United States Environmental Protection Agency. Web site. www.epa.gov/criteria-air-pollutants/naaqs-table
8. Lee BJ, Kim B, Lee K. Air pollution exposure and cardiovascular disease. Toxicol Res. 2014; 30: 71-75.
9. Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011; Article ID 487074.
10. Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008; 122: 456-468.
11. Kim JW, Park S, Lim CW, Lee K, Kim B. The role of air pollutants in initiating liver disease. Toxicol Res. 2014; 30(2): 65–70.
12. Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004; 16(6-7): 437-45.
13. Franken C, Lambrechts N, Govarts E, et al. Phthalate-induced oxidative stress and association with asthma-related airway inflammation in adolescents. Int J Hyg Environ Health. 2017; 220: 468-477.
14. Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC.Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health. 2017; 16(1): 42.
15. WHO guidelines for indoor air quality: selected pollutants. Web site. http://www.euro.who.int/ data/assets/pdf_file/0009/128169/e94535.pdf
16. Brook RD, Rajagopalan S, Pope CA 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010; 121(21): 2331-78.
17. Piantadosi CA. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic Biol Med. 2008; 45(5): 562-9.
18. Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008; 26(4): 339-62.
19. Jeng HA. Chemical composition of ambient particulate matter and redox activity. Environ Monit Assess.2010; 169(1-4): 597-606.
20. Li N, Sioutas C, Cho A. et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003; 111(4): 455-60.
21. Li N, Hao M, Phalen RF, Hinds WC, Nel AE. et al. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. ClinImmunol. 2003; 109(3): 250-65.
22. Meng H, Xia T, George S , Nel AE. A predictive toxicological paradigm for the safety assessment of nanomaterials. ASC Nano. 2009; 3(7): 1620-7.
23. Kloog I, Coull BA, Zanobetti A, Koutrakis P, Schwartz JD. Acute and chronic effects of particles on hospital admissions in New-England. Plos One. 2012; 7(4): e34664.
24. Berglund D. Long-Term Effects of Air Pollution. WJM. 1996; 165(3): 140. www.ncbi.nlm.nih.gov/pmc/articles/PMC1303722/pdf/westjmed003480042a.pdf
25. Vigotti MA. Short-term effects of exposure to urban air pollution on human health in Europe. The APHEA Projects (Air Pollution and Health: a European Approach). Epidemiol Prev. 1999; 23(4): 408-15.
26. Lü J, Liang L, Feng Y, Li R, Liu Y. Air Pollution Exposure and Physical Activity in China: Current Knowledge, Public Health Implications, and Future Research Needs. Int J Environ Res Public Health. 2015; 12(11): 14887-97.
27. Péter S, Holguin F, Wood LG. et al. Nutritional Solutions to Reduce Risks of Negative Health Impacts of Air Pollution. Nutrients. 2015; 7(12): 10398-416.
28. Brook RD. Cardiovascular effects of air pollution. ClinSci (Lond). 2008; 115(6): 175-87.
29. Liu L, Poon R, Chen L, et al. Acute effects of air pol- lution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009; 117(4): 668-74.
30. Chang YK, Wu CC, Lee LT, Lin RS, Yu YH, Chen YC. The short-term effects of air pollution on adolescent lung function in Taiwan. Chemosphere. 2012; 87(1): 26-30.
31. McCreanor J, Cullinan P, Nieuwenhuijsen MJ. et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007; 357(23): 2348-58.
32. Fortoul TI, Rojas-Lemus M, Rodriguez-Lara V et al. Air pollution and its effects in the respiratory system. In: The Impact of Air Pollution on Health, Economy, Environment and Agricultural Sources, Dr. Mohamed Khallaf (Ed.), InTech, 2011. DOI: 10.5772/17766.
33. Salvi S. Health effects of ambient air pollution in children. Paediatr Respir Rev. 2007; 8(4): 275-80.
34. Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003; 60(8): 612-6.
35. Chen C, Arjomandi M, Tager IB, Holland N, Balmes JR. Effects of antioxidant enzyme polymorphisms on ozone-induced lung function changes. Eur Respir J. 2007; 30(4): 677-83.
36. Fagerås Böttcher M, Hmani-Aifa M, Lindström A. et al. A TLR4 polymorphism is associated with asthma and reduced lipopolysaccharide-induced interleukin-12(p70) responses in Swedish children. J Allergy ClinImmunol. 2004; 114(3): 561-7.
37. Li YF, Gauderman WJ, Avol E, Dubeau L, Gilliland FD. Associations of tumor necrosis factor G-308A with childhood asthma and wheezing. Am J Respir Crit Care Med. 2006; 173(9): 970-6.
38. David GL, Romieu I, Sienra-Monge JJ et al. Nico- tinamide adenine dinucleotide (phosphate) reduced: quinone oxidoreductase and glutathione S-transferase M1 polymorphisms and childhood asthma. Am J Respir Crit Care Med . 2003; 168(10): 1199-204.
39. Masuko H, Sakamoto T, Kaneko Y. et al. An interaction between Nrf2 polymorphisms and smoking status affects annual decline in FEV1: a longitudinal retrospective cohort study. BMC Med Genet. 2011; 20; 12: 97.
40. De Prins S, Dons E, Van Poppel M. et al. Airway oxidative stress and inflammation markers in exhaled breath from children are linked with exposure to black carbon. Environ Int. 2014; 73: 440-6.
41. Rosa MJ, Yan B, Chillrud SN. et al. Domestic airborne black carbon levels and 8-isoprostane in exhaled breath condensate among children in New York City. Environ Res. 2014; 135: 105-10.
42. Patel MM, Chillrud SN, Deepti KC, Ross JM, Kinney PL. et al. Traffic-related air pollutants and exhaled markers of airway inflammation and oxidative stress in New York City adolescents. Environ Res. 2013; 121: 71-8.
43. Huang W, Wang G, Lu SE. et al. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med. 2012; 186(11): 1150-9.
44. Li W, Wilker EH, Dorans KS. et al. Short-Term Expo- sure to Air Pollution and Biomarkers of Oxidative Stress: The Framingham Heart Study. J Am Heart Assoc. 2016; 28, 5(5).
45. Tsukagoshi H, Kawata T, Shimizu Y, Ishizuka T, Dobashi K, Mori M. 4-Hydroxy-2-nonenal enhances fibronec- tin production by IMR-90 human lung fibroblasts partly via activation of epidermal growth factor receptor-linked extracellular signal-regulated kinase p44/42 pathway. Toxicol Appl Pharmacol. 2002; 184(3): 127-35.
46. Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Toxicol Appl Pharmacol. 1998; 150(2): 338-49.
47. Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Induction of inflammatory mediators in Lipid ozonation products activate phospholipases A2, C, and D. human airway epithelial cells by lipid ozonation products. Am J Respir Crit Care Med. 1999; 160(6): 1934-42.
48. Taggart C, Cervantes-Laurean D, Kim G. et al. Oxida- tion of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem. 2000; 275: 27258-27265.
49. Mikerov AN, Umstead TM, Gan X. et al. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol. 2008; 294(1): L121-30
50. Manzanares D, Rodriguez-Capote K, Liu S. et al. Mo- dification of tryptophan and methionine residues is implicated in the oxidative inactivation of surfactant protein B. Biochemistry. 2007;46(18): 5604-5615.
51. Rodríguez-Capote K, Manzanares D, Haines T, Poss- mayer F. Reactive oxygen species inactivation of surfactant involves structural and functional alterations to surfactant proteins SP-B and SP-C. Biophys J. 2006; 90(8): 2808-2821.
52. Starosta V, Griese M. Oxidative damage to surfactant protein D in pulmonary diseases. Free Radic Res. 2006; 40(4): 419-425.
53. Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013; 10(9): 3886-3907.
54. Møller P, Folkmann JK, Forchhammer L. et al. Air pollution, oxidative damage to DNA, and carcinogenesis. Cancer Lett. 2008; 266(1): 84-97.
55. Tovalin H, Valverde M, Morandi MT, Blanco S, White- head L, Rojas E. DNA damage in outdoor workers occupationally exposed to environmental air pollutants. Occup Environ Med. 2006; 63(4): 230-236.
56. Prahalad AK, Inmon J, Dailey LA, Madden MC, Ghio AJ, Gallagher JE. Air pollution particles mediated oxi- dative DNA base damage in a cell free system and in human airway epithelial cells in relation to particulate metal content and bioreactivity. Chem Res Toxicol. 2001; 14(7): 879-887.
57. Tokiwa H, Sera N, Nakanishi Y, Sagai M. 8-Hydroxy- guanosine formed in human lung tissues and the association with diesel exhaust particles. Free Radic Biol Med. 1999; 27(11-12): 1251-1258.
58. Kawanishi S, Hiraku Y, Murata M, Oikawa S. The role of metals in site-specific DNA damage with reference to carcinogenesis. Free Radic Biol Mec. 2002;32(9): 822-832.
59. Cheng TJ, Kao HP, Chan CC, Chang WP. Effects of ozone on DNA single-strand breaks and 8-oxoguanine formation in A549 cells. Environ Res. 2003; 93(3): 279-284.
60. Ito K, Inoue S, Hiraku Y, Kawanishi S. Mechanism of site-specific DNA damage induced by ozone. Mutat Res. 2005; 585(1-2): 60-70.
61. Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol. 2010; 588(Pt 3): 423-433.
62. Barker JS, Wu Z, Hunter DD, Dey RD. Ozone exposure initiates a sequential signaling cascade in airways involving interleukin-1beta release, nerve growth factor secretion, and substance P upregulation. J Toxicol Environ Health A. 2015; 78(6): 397-407.
63. Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001; 125(1-2): 47-65.
64. Health Effects of Ozone in the General Population. United States Environmental Protection Agency. Web site. www.epa.gov/ozone-pollution-and-your-patients-health/health-effects-ozone-general-population
65. Schelegle ES, Walby WF. Vagal afferents contribute to exacerbated airway responses following ozone and allergen challenge. Respir Physiol Neurobiol. 2012; 181(3): 277-285.
66. Lee LY, Widdicombe JG. Modulation of airway sensitivity to inhaled irritants: role of inflammatory mediators. Environ Health Perspect. 2001; 109 Suppl 4:585-589.
67. McKinnon KP, Madden MC, Noah TL, Devlin RB. In vitro ozone exposure increases release of arachidonic acid products from a human bronchial epithelial cell line. Toxicol Appl Pharmacol. 1993; 118(2): 215-223.
68. Leikauf GD, Simpson LG, Santrock J. et al. Airway epithelial cell responses to ozone injury. Environ Health Perspect. 1995; 103 Suppl 2: 91-95.
69. Du Y, Xu X, Chu M, Guo Y, Wang J. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis. 2016; 8(1): E8-E19.
70. Bind MA, Baccarelli A, Zanobetti A, et al. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology. 2012; 23(2): 332-340.
71. Chen R, Meng X, Zhao A. et al. DNA hypomethylation and its mediation in the effects of fine particulate air pollution on cardiovascular biomarkers: A randomized crossover trial. Environ In. 2016; 94: 614-619.
72. Wang C, Chen R, Cai J. et al. Personal exposure to fine particulate matter and blood pressure: A role of angiotensin converting enzyme and its DNA methylation. Environ Int. 2016; 94: 661-666.
73. Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002; 105, 1543-1536.
74. Urch B, Silverman F, Corey P. et al. Acute blood pres- sure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005; 113, 1052-1055.
75. Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am J Epidemiol. 2001; 154, 758-764.
76. Salvi S, Blomberg A, Rudell B. et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999; 159, 702-709.
77. Mukae H, Vincent R, Quinlan K. et al. The effect of repeated exposure to particulate air pollution (PM10) on the bone marrow. Am J Respir Crit Care Med. 2001; 163, 201-209.
78. Goto Y, Ishii H, Hogg JC. et al. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am J Respir Crit Care Med. 2004; 170, 891-897.
79. Schwartz J, Park SK, O‘Neill MS, et al. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005; 172, 1529-1533.
80. Franchini M, Guida A, Tufano A, Coppola A. Air pollution, vascular disease and thrombosis: linking clinical data and pathogenic mechanisms. J Thromb Haemost. 2012; 10, 2438-2451.
81. Lucking AJ, Lundback M, Mills NL, et al. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J. 2008; 24, 3043-3051.
82. Mills NL, Törnqvist H, Robinson SD, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Cirulation. 2005; 112, 3930-3936.
83. Kilinç E, Van Oerle R, Borissoff JI, et al. Factor XII acti- vation is essential to sustain the procoagulant effects of particulate matter. J Thromb Haemost. 2011; 9, 1359-139

