Diabetes in Children and the Role of Micronutrients
The steady increase in diabetes among children and young adults is one of the emerging health problems worldwide.
This disease, characterized by impaired glucose metabolism and abnormally high blood glucose levels, has serious negative health consequences in young organisms, many of which surface later in life.
In addition, pharmacological treatments primarily developed and tested in adult diabetics carry health risks and are associated with unanticipated side effects when administered to children.
All these concerns generate interest in developing micronutrient based approaches as safe and effective alternatives to medical drugs in both prevention and management of diabetes, especially in children.
This review outlines the scope of this health problem, highlights current therapeutic approaches to pediatric diabetes, and presents the latest findings on preventive and therapeutic potential of micronutrients and other natural compounds in controlling diabetes in children.
Introduction
Diabetes is a metabolic disorder characterized by high blood glucose levels resulting from either insufficient insulin production in the pancreas, inadequate response of the body’s cells to insulin, or both. More than 200 million people suffer from diabetes worldwide, and, according to the World Health Organization (WHO), it will become the seventh leading cause of death by 20301. Especially alarming is the growing number of children and adolescents diagnosed with this condition, which has skyrocketed within the last 20 years, prompting the journal Diabetes Care to call it an “emerging epidemic”.
Type 1 diabetes
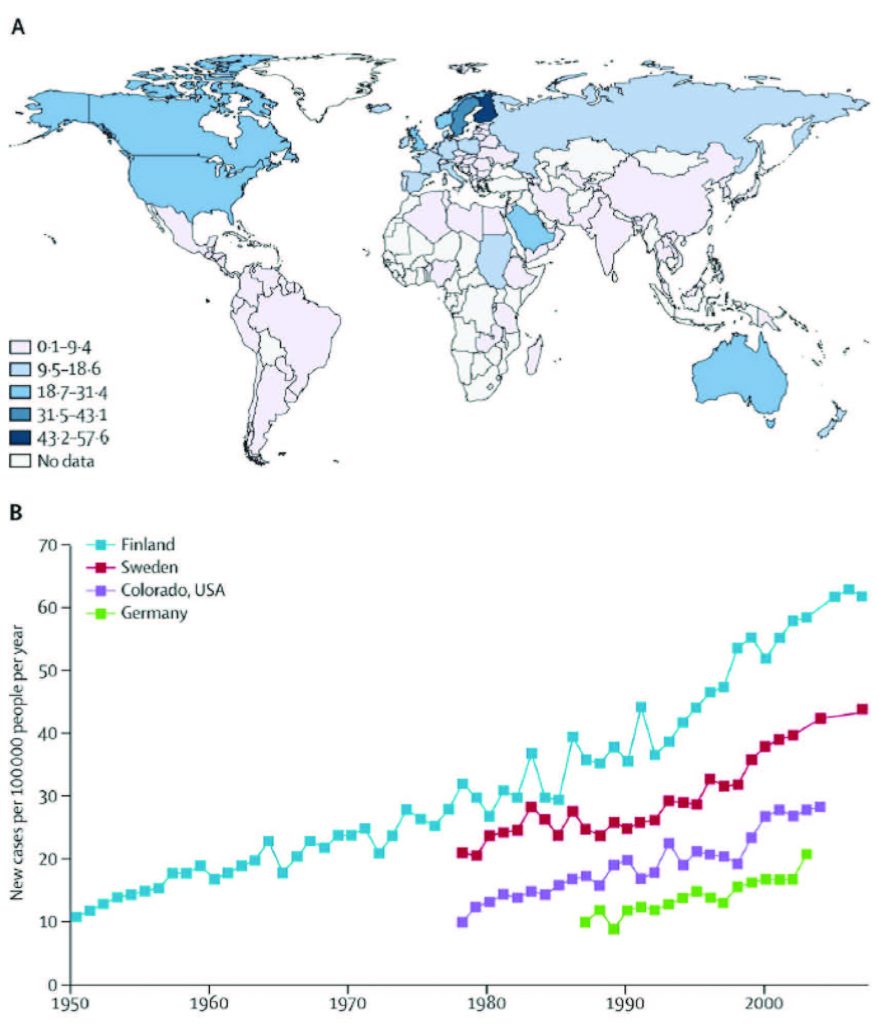
Type 1 diabetes (known as insulin-dependent, juvenile or childhood-onset diabetes) is characterized by deficient insulin production in the pancreas,which requires daily administration of insulin. Symptoms include frequent urination, thirst, persistent hunger, weight loss, vision changes and fatigue, all of which may occur suddenly2. Until not long ago, most children diagnosed with diabetes suffered from the type 1 form of the disease. Its incidence and prevalence had varied depending on the geographical region (Figure 1) and it was speculated that environmental factors could contribute to these differences3.
According to these data, type 1 diabetes is widespread among children in Finland and Sardinia and rather uncommon in China, India and Venezuela. Wide variations in disease occurrence have also been noted between neighboring areas in Europe and in North America. Finland, Germany and Norway have reported annual increases of type 1 diabetes in children. Barat reports that in France currently more than one quarter of children diagnosed with type 1 diabetes are under the age of 54.
Continuing increase in type 1 diabetes incidence was also registered in the UK, where the disease was shown to be more frequent in children than in young adults5.
In Europe, in general, the substantial increase in type 1 diabetes has been noted in children younger than 5 years of age3.
Forecasts indicate that a doubling of new cases of type 1 diabetes in European children younger than 5 years is predicted between 2005 and 2020, and that the incidence in children younger than 15 years will rise by 70%6.
Type 2 diabetes
Type 2 diabetes (also called non-insulin-dependent or adult-onset diabetes) results from the body’s ineffective response to insulin or its insufficient production. About 90% of people diagnosed with diabetes suffer from type 22. Although symptoms may be similar to type 1 diabetes, they are often less pronounced. As a result, the disease may be diagnosed several years after onset, once its complications have already arisen.
Until recently, this type of diabetes was seen mainly in adults, but now it is increasingly diagnosed in children2. Experts estimate that type 2 diabetes in youth has grown from less than 5 percent in 1994 to about 20 percent of all newly diagnosed cases in recent years. Currently, about 45% of diabetes cases in adolescents are attributed to type 2 diabetes7,8.
Type 1 diabetes
The exact causes of type 1 diabetes are largely unknown. Since type 1 diabetes is often inherited, it was speculated that genetics may play a pivotal role in its development. In most cases it is a result of an autoimmune condition in which the immune system damages the pancreatic cells which consequently cease producing a hormone, insulin. Other anticipated causes include:
Childhood vaccinations. The U.S. Centers for Disease Control (CDC) supports scientific studies by Classen, whose research concludes that vaccines can trigger type 1 diabetes. The evaluation of type 1 diabetes cases in relation to immunizations indicate an increase in the incidence of type 1 diabetes 2-4 years following the introduction of the MMR (measles, mumps, and rubella) and pertussis vaccines. Inversely, a decrease in type 1 diabetes was observed 3-4 years after the discontinuation of pertussis and BCG (Bacillus Calmette–Guérin) vaccines9. Classen and colleagues also evaluated whether Hemophilus influenza B (HiB) vaccine can be associated with an increased risk of type 1 diabetes. About 116,000 children born in Finland (October 1st, 1985 – August 31st, 1987) were randomized to receive 4 divided doses of the HiB vaccine (PPR-D, Connaught) starting at 3 months of life, or one dose starting after 24 months of life. A control-cohort included 128,500 children born in Finland in the 24 months prior to the HiB vaccine study. The results showed that exposure to HiB immunization was associated with an increased risk of type 1 diabetes10.
Low levels of vitamin D. The association between vitamin D deficiency and type 1 diabetes was studied in 185 children (9.8 years old on average) diagnosed with this disease. The subjects included 51% Caucasian, 25% Hispanic, 4% mixed-Hispanic, 4% African American, and 16% other/mixed race. The majority of these young diabetic patients had low vitamin D levels (40% showing vitamin D insufficiency, and 18% were vitamin D deficient). The study concluded that vitamin D deficiency is common at onset of pediatric type 1 diabetes11. These findings corroborated with a metaanalysis study by Liu and colleagues, which concluded that serum vitamin D was significantly lower in children with type 1 diabetes than in healthy controls12.
Type 2 diabetes
Development of type 2 diabetes has been largely associated with a global rise of childhood obesity. Numerous data show that healthy eating and lifestyle habits are a strong defense against this disease13.
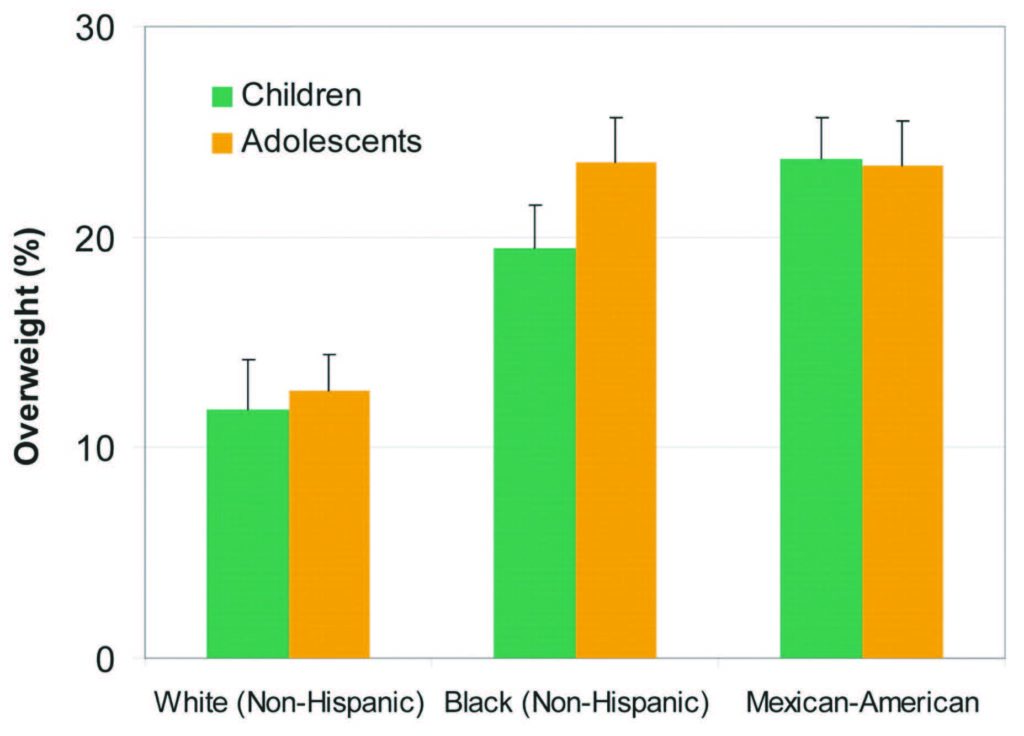
Overweight. The increase in type 2 diabetes among children and adolescents has emerged in parallel with an alarming rise in overweight and obesity among youth (Figure 2). Increase in visceral fat has been implicated in developing the insulin resistance which often precedes type 2 diabetes14. A prospective cohort study conducted between 1965 and 2007 in 5,532 American Indian children showed that diabetes incidence was higher in overweight than in normal weight children15. These results, which corroborate data obtained in other populations and countries (WHO European Region), highlight the strong involvement of body weight and dietary habits in the development of diabetes in children.
Diet and food industry. The food industry has an important role in influencing children’s diets and their dietary choices. Due to food processing methods and modern agriculture our diet has become calorie rich and micronutrient poor. In addition, most processed foods advertised are targeted at and consumed by the young population and contain large amounts of sugar (i.e. soft drinks, cereals, snacks and ready-toeat meals), increasing the risk of diabetes.
Boulton and colleagues investigated the amount of sugars in juice drinks and smoothies marketed to children sold by seven major UK supermarkets. The results showed that the sugar content in these products is unacceptably high and the study concluded that manufacturers must stop adding unnecessary sugars and calories16 as a necessary measure to reduce the prevalence of overweight, obesity and type 2 diabetes17. Especially of concern is an increasing consumption of High Fructose Corn Syrup (HFCS), which, as an inexpensive ingredient, has become the most commonly used sweetener in processed foods. Its introduction parallels a rise in obesity, diabetes, hypertension and kidney disease18,19, as the liver converts fructose into fat. It has been shown that as little as four weeks of a moderate fructose diet can increase blood cholesterol and glucose levels. Human and animal studies have shown that fructose can induce metabolic syndrome including insulin resistance, coronary microvascular disease, and oxidative stress. Interestingly these effects have not been seen in animals fed glucose or starch, which suggests that these mechanisms are not mediated by excessive caloric intake, but rather relate to fructose processing in the body20.
Physical activity. The WHO defines “physical activity” as any bodily movement involving skeletal muscles that requires energy expenditure. This differs from “exercise”, which is a physical activity that is planned and structured. Insufficient physical activity is considered one of the 10 leading risk factors for death worldwide and a key risk factor for diabetes. According to the WHO, over 80% of the world’s adolescent population has insufficient physical activity21,22.
Insufficient physical activity has also been linked to a higher risk of diabetes in children. A retrospective population-based survey in 2,720 adults (1,096 male and 1,624 female) showed that early sport practice during childhood and adolescence was associated with lower occurrence of type 2 diabetes and arterial hypertension later in adulthood23. These data are supported by a prospective longitudinal study in 8 year old children (199 subjects) and in 12 year olds (107 children). The evaluation of anthropometric data, blood tests and physical activity in this population showed that physical activity improves insulin sensitivity and over time affects the levels of C-peptide (indicator of insulin production in the pancreas)24.
Health consequences of type 1 and type 2 diabetes in children
Children develop type 2 diabetes at a later age compared to those with type 1 diabetes. Both types of diabetes can be initially asymptomatic and early diagnosis of this disease is critical for minimizing its negative health effects later in life.
Hyperglycemia. Hyperglycemia is characterized by a high level of glucose in the blood, which causes biological damage by attaching to other molecules, often proteins such as the ones that build our blood vessels or transport oxygen (hemoglobin). This glucose-triggered damage contributes to cellular dysfunction and various other complications of diabetes25.
Cardiovascular disease. Although diabetic children and adolescents with type 1 and type 2 diabetes can be asymptomatic for cardiovascular disease, the long term effects of high blood glucose levels result in detrimental health effects when they become adults. Circulatory problems and impaired blood flow can lead to blindness from clots formed in the arteries of the eyes, cause kidney failure, gangrene, heart attacks from blockages developing in the coronary arteries, and strokes from obstructing blood flow to the brain. Aglycoprotein involved in bone metabolism, Osteoprotegerin (OPG), has emerged as an independent biomarker of cardiovascular disease and is implicated in diabetes and poor glycemic control. A study in 56 children (12.1 ± 3.4 years old) with type 1 diabetes, and in 46 healthy children (11.3 ± 3.0 years old), showed that serum OPG levels were significantly elevated in children with type 1 diabetes compared to healthy controls26. In a Japanese study, approximately 10% of subjects who developed type 2 diabetes before the age of 30 had atherosclerotic vascular disease, poor glycemic control and microvascular complications, especially microalbuminuria, nephropathy, and end-stage renal disease. It has been shown that individuals with early onset type 2 diabetes have an increased tendency to develop microalbuminuria and hypertension, the known risk factors for cardiovascular disease27. In addition, abnormal lipoprotein plasma levels have been observed in children and adolescents with type 1 and 2 diabetes28,29 as well as a risk of chronic inflammation30.
Celiac disease. Most patients with type 1 diabetes have asymptomatic celiac disease or display symptoms that may be confused for diabetes. Studies in Western Europe, North America, and Australia indicate that the prevalence of celiac disease among children and adults with type 1 diabetes greatly exceeds this condition found in the general population. For these reasons all children with type 1 diabetes should be evaluated for celiac disease31.
Chronic autoimmune thyroiditis. This condition is characterized by thyroid dysfunction and the presence of thyroid specific autoantibodies in blood serum. The autoimmune thyroiditis appears to be significantly frequent among young patients with type 1 diabetes. Since 1990, Kourdonouri and collaborators have been conducting annual screenings for thyroid disease in 659 children and adolescents (54.3% were boys) with type 1 diabetes. This included testing for antibodies against thyroperoxidase (anti-TPO), thyroglobulin (anti-TG) and TSH (thyroid stimulating hormone). The study showed that all patients with significantly higher values for anti-TPO and anti-TG at the onset of type 1 diabetes remained positive during the following five years. The authors concluded that anti-TPO and TSH should be evaluated in children with type 1 diabetes at onset of this disease and that children older than 12 should be screened yearly32. These data have been confirmed by a recent study in Taiwanese children and adolescents with type 1 diabetes, which showed that autoimmune thyroid disorders were quite common among diabetics33.
Cognitive and behavioral impairment. Children and adolescents with type 1 diabetes34 (Figure 3) and type 2 diabetes often display behavioral disorders and cognitive impairment. Jabbourand colleagues investigated barriers to active lifestyles in children with type 1 diabetes. They showed that among others a loss of control of diabetes, fear of hypoglycemia, work schedule, and low fitness levels were the most important barriers that affected children’s psychological balance35. A group of researchers at the Cincinnati Children’s Hospital Medical Center assessed cognitive and behavioral performance in obese adolescents with type 2 diabetes and found they performed worse than controls36.
Diabetic nephropathy. Diabetic nephropathy is defined as an abnormal urinary albumin excretion (macroalbuminuria). Suh and colleagues found that early stages of nephropathy in pediatric patients with type 1 diabetes can be detected by urinary tubular damage and inflammatory markers37. A study in 56 patients with type 1 diabetes (mean age 13.1) and 49 healthy controls (mean age 12.8) evaluated serum NGAL (Neutrophil Gelatinase-Associated Lipocalin) and cystatin C (nephelometry), in addition to standard blood chemistry and urinary albumin excretion, at enrollment and after 12-15 months. The researchers suggested that NGAL and cystatin C, which are the markers of renal injury, may be used as supplementary tests to the urine albumin excretion for early detection of renal dysfunction in children38. Between 2002 and 2013, the annual prevalence of diabetic nephropathy in pediatric patients with diabetes increased from 1.16 to 3.44% for all cases and was highest in patients aged 12 to 18 years of age39,40.

Figure 3. Short and long term behavioral and cognitive reported alterations following type 1 diabetes mellitus onset in children and adolescents according to type 1 diabetes mellitus duration34.
Diabetic neuropathy. Nerve damage represents the major complication in type 1 diabetes classified as polyneuropathy, focal neuropathy and autonomic neuropathy. The latter seems to be detectable even in asymptomatic children and adolescents with diabetes and is associated with the most serious consequences, such as unawareness of hypoglycemia and cardiovascular dysfunction41.
Diabetic retinopathy. Diabetic retinopathy is caused by damage to the blood vessels of the tissue at the back of the eye (retina), which at its early stages does not cause symptoms. A retrospective study of 143 children aged 12 or younger concluded that retinopathy screening should start at this young age42.
Juvenile arthritis. Juvenile arthritis is caused by inflammation of the joints. It has been shown that juvenile arthritis and diabetes end-points were considerably larger in pediatric patients with type 1 diabetes than in the general population43.
Pediatric osteoporosis. Pediatric osteoporosis is characterized by low bone mass density (BMD). While in adults low BMD is generally due to net bone loss after its peak accrual, in children it can result from loss of bone or, more commonly, impaired bone mineralization. Endocrine disorders, such as diabetes can lead to pediatric osteoporosis44.
Periodontal disease. Periodontal diseases are bacterial infections of the tissues surrounding and supporting the teeth. Gingivitis, an inflammation of the soft tissues only, can progress to periodontitis, where destruction of the connective tissue attachment and alveolar bone can eventually lead to tooth loss. Periodontal destruction in diabetes can start early in life and become more prominent as children become adolescents45. Xavier et al. evaluated 168 children (13 +/- 3.5 years old) with type 1 diabetes for plaque index (PI), sites with bleeding on probing (BOP), probing depth (PD) and clinical attachment level (CAL) in all occlusion of permanent teeth. Gingivitis was diagnosed in 20.8% children and 5.9% children had periodontitis. The authors concluded that periodontal problems in this young population were significantly associated with the duration of type 1 diabetes and poor glycemic control46.
Skin problems. Diabetes can affect every part of the body, including the skin. In fact, skin problems are sometimes the first sign of diabetes. Some of them, such as bacterial infections, fungal infections, and itching can affect anyone, but people with diabetes are more prone to them. Skin problems that develop mostly or exclusively in people with diabetes include diabetic dermopathy, necrobiosis lipoidicadiabeticorum, diabetic blisters, and eruptive xanthomatosis47.
Digestive tract infections: Studies have shown frequent yeast-like fungi infections (e.g. Candida albicans) in the digestive tract of children with type 1 diabetes48, which is resistant to conventional antifungal treatment49 (Figure 4).
Diagnosis and conventional treatments of diabetes in children
Children are diagnosed with diabetes when blood plasma glucose values at fasting are at or above 126 mg/dl. Another blood test (called HbA1) measures the percentage of blood sugar attached to hemoglobin, the oxygen-carrying protein in red blood cells. This test reflects an average blood sugar level for the past two to three months. An A1C level of 6.5 percent or higher on two separate tests indicates diabetes. In order to distinguish type 1 and type 2 diabetes, a child can also get a C-peptide test or autoantibodies test, which detects the presence of specific antibodies (ZnT8Ab), characteristic for only type 1 diabetes.
Conventional treatments
Insulin and metformin
The U.S. Food and Drug Administration (FDA)50 has only approved the use of metformin and insulin in children (FDA). Insulin is administered in children with type 1 diabetes, while insulin together with metformin can be prescribed to children with type 1 and type 2 diabetes.
Insulin. The first option in the treatment of type 1 diabetes is a hormone, insulin, which regulates serum glucose by increasing its uptake into muscle and adipose tissue and decreases glucose production in the liver. The following insulin products are indicated for the use in children: Aspart, Glulisine, Lispro, Detemir, and Glargine51.
The administration of insulin is not risk-free as it is associated with a risk of hypoglycemia, peripheral hyperinsulinemia, headache, and weight gain. A recent Italian study which investigated the effects of Detemir in 15 pre-pubertal children with type 1 diabetes showed that this treatment was associated with an abnormal body weight not related to pubertal growth52.
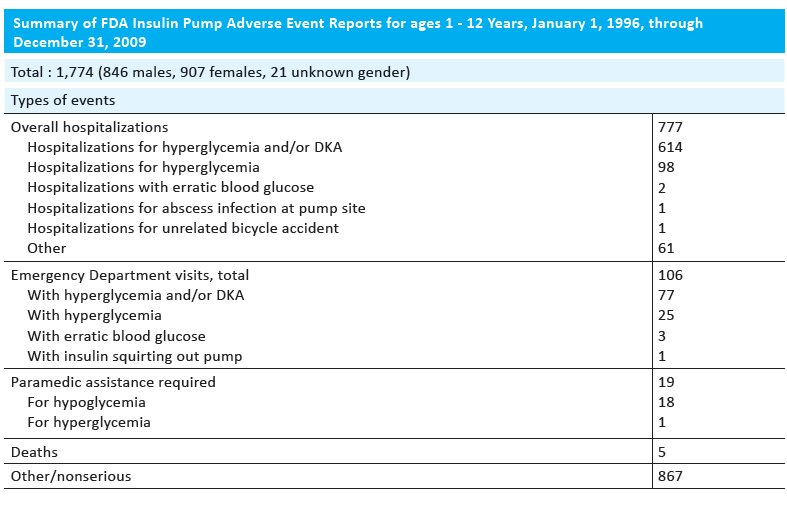
The delivery of insulin not by injection, but by a pump, is often encouraged in children and adolescents. Pumps deliver insulin 24 hours a day through a catheter placed under the skin.This is more convenient for a patient and allows for better control of blood sugar. However, there were reports of pump-related adverse events in children 1–12 years old. From January 1, 1996, through December 31, 2009, a total of 21,769 reports were collected for all ages. Of these, 1,774 were for children aged 1–12 years and more than half resulted in serious outcomes (Table 1). Children were hospitalized for hyperglycemia or hypoglycemia and deaths of five patients were reported53.
Table 1. Summary of FDA insulin pump adverse event reports for ages 1-12 years, January 1, 1996, through December 31, 200953.
Metformin. Metformin, a drug classified as a biguanide, is the first option in the treatment of type 2 diabetes in pediatrics. It works by reducing glucose production and activating glucose uptake in peripheral tissues. The administration of metformin can cause various side effects, such as decreased appetite, diarrhea, muscle pain, and gastrointestinal problems54.
Metformin together with insulin has also been used in the treatment of type 1 diabetes. In a study in 140 overweight adolescents with type 1 diabetes the use of metformin as an adjunct to insulin did not improve glycemic control and led to an increased risk of gastrointestinal adverse events55. Another study investigated the prevalence of chronic diarrhea in 861 patients with type 1 and type 2 diabetes taking metformin. The authors concluded that chronic diarrhea is often associated with diabetes and the most common cause of non-diabetic diarrhea is metformin56.
Other conventional therapies
Although the FDA has only approved metformin and insulin in diabetic children and adolescents, some physicians recommend the use of drugs which have been approved for adults57. These often lack clinical evidence of their efficacy in a pediatric population, which makes young patients prone to unexpected health risks51. A short review of these treatment options is presented below:
Thiazolidinediones(TZDs). This class of drugs, which has not been approved in children, works by increasing insulin sensitivity in the cells. Wen and colleagues examined the potentially inappropriate prescription of TZDs in different groups of patients, including young people under 18 years of age. Data collected from Taiwan’s National Health Insurance data set from 2001 to 2006 showed that inappropriate prescription of TZDs actually increased from 9.41% in 2001 to 12.50% in 200658. The use of TZDs decreased after a black-box warning was issued in 2007 for a drug, rosiglitazone, highlighting its cardiovascular risks59.
Recently, the short-term use of rosiglitazone was evaluated in a pilot study in 21 obese adolescents (13 to 18 years old) with impaired glucose tolerance. The study found that 58% of patients treated with rosiglitazone returned to normal glucose tolerance, compared to 44% of patients taking placebo. Although few adverse events surfaced during this treatment, the authors concluded that, given the cardiovascular concerns with rosiglitazone in adults and the small pool of data on its use in children, it would be premature to recommend rosiglitazone in children60.
Sulfonylureas. Sulfonylureas promote insulin secretion by acting on pancreatic β cells60. They are not labeled for pediatric use and data on their use in children are limited.
The University Group Diabetes Program clinical trial has reported an association between the administration of the sulfonylurea drug, tolbutamide, and increased cardiovascular mortality. This provided the basis for the FDA warning about “increased risk of cardiovascular mortality” for this and other oral sulfonylurea hypoglycemic drugs61.
Amylin analog (Pramlintide). It is a synthetic analog of human amylin, a hormone that acts together with insulin to delay gastric emptying and inhibit release of glucagon, a hormone that raises the level of glucose in the blood. According to the FDA this drug has not been sufficiently studied in children but some doctors apply it off-label. Clinical evidence on the use of pramlintide in children is limited to type 1 diabetes and to small studies enrolling only 8 to 13 patients. In one of the studies, 10 adolescents 13 to 17 years of age were randomized to active treatment with 15 mcg of pramlintide before meals (titrated to 30 mcg if tolerated) or to control for 28 days. This preliminary study led to improvements in HbA1c, body weight, and insulin dose in adolescents with type 1 diabetes. However, the package insert for this drug contains a black box warning regarding risk of severe hypoglycemia in type 1 diabetes. The most common adverse events include hypoglycemia, nausea, headache, anorexia, and abdominal pain60.
Other drugs, such as Incretin based therapies: dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists as well as Meglitinides and Alpha glucosidase inhibitors were not approved for use in children60.
Differences in metabolizing drugs in children and adults
Limited data are available on age-related drug metabolism and behavior, especially in the pediatric population which encompasses wide age ranges, from the neonates (up to 1 month) to adolescents (12-16 years). Most diabetic drugs prescribed for children have been tested only in adults and their dosage is adjusted to the child´s body weight. This, however, does not reflect profound changes in body’s composition, physiology and biochemistry occurring in a growing organism, all of which have a profound impact on drug absorption, bioavailability and metabolism as well as their physiological effects. Therefore, use of these drugs in pediatrics carry unexpected health risks, some of which may surface later in life. There are several factors that affect absorption and metabolism of pharmaceutical drugs in children.
Hepatic function. The liver plays an important role in drug metabolism and its function changes at different stages of human development. As such, there are differences in metabolizing drugs in children, adults, and the elderly. Most oral drugs, which are chemical substances not known to the body, undergo a specific metabolic detoxification route in the liver. After they are absorbed in the gastrointestinal tract they do not immediately reach systemic circulation, but enter the liver for so called first-pass metabolism. There, they are processed and modified in a chain of metabolic reactions rendering drugs less toxic so they can enter the blood circulation to be distributed and delivered to the target tissue. Infants have impaired first-pass metabolism of drugs62, which makes drugs more bioavailable and not properly processed, resulting in an increased risk of drug-induced side effects.
An important enzyme system involved in drug metabolism and detoxification involves cytochromes P-450 (CYP450). This metabolic activity varies with age as supported by Hines´ investigation on ontogeny of human hepatic cytochromes P450. He measured six key cytochromes P450 in 240 human liver samples, representing ages from 8 weeks gestation to 18 years. The results showed a wide range of age-related variations in enzyme activities for each cytochrome group, which indicates different ability of our body from infancy through adolescence in processing drugs and other toxins63.
Pancreatic function. The pancreas is composed of two functional components: the “exocrine pancreas” which is built by the cells that produce enzymes, and the “endocrine pancreas” where the islets of Langerhans produce the hormones insulin and glucagon involved in glucose homeostasis. Unlike the liver, the “exocrine pancreas” has been considered to make insignificant contribution to drug metabolism and detoxification, therefore, it is seldom identified as a target organ of xenobiotic toxicity64. For these reasons little information is available for the enzymatic pattern of the pancreas65. However, it is known that infants have decreased pancreatic exocrine function, which matures by the first year62. Such physiological evolution makes infants more susceptible to druginduced side effects and pancreas impairment. When the exocrine pancreas becomes a target organ of xenobiotic toxicity, serious clinical implications can affect pancreatic cells64.
Gastric function. Gastric pH and its emptying time vary with age. Premature infants have impaired gastric acid production until after 32 weeks of gestational age. This allows orally administered acid-labile drugs that may not be absorbed intact in the mature individual to have greater bioavailability in premature and very young infants62.
Gut function. As with the skin area, the absorptive surface area of the gut is relatively greater in the infant than in the adult. The immature gut also exhibits permeability to large molecular species, including intact proteins that are not absorbed by the mature gut. Gastric emptying and gut transit time may be prolonged in premature and ill newborns. On the other hand, healthy infants may have transit times shorter than those for adults62.
Micronutrients and diabetes
Micronutrients, which include vitamins, minerals and other natural compounds, play an essential role in human nutrition in supporting optimum growth and development and sustaining life. Although they are required in much smaller amounts than proteins, carbohydrates and fats, they enable our body to convert food to bioenergy, facilitate synthesis of enzymes and hormones, build cellular compounds, and sustain all physiological functions. Micronutrient deficiencies, especially long-term, are associated with severe health consequences and even death66.
Micronutrients, essential for healthy cellular metabolism, including proper processing of drugs and toxins, are safe at a wide dosage ranges. Although a “healthy” diet can theoretically provide adequate amounts of nutrients, in reality many people fail to meet the required levels. The risk of developing micronutrient deficiencies is higher in pathological conditions, such as diabetes, due to high demands for micronutrients in response to the body’s metabolic changes as well as to anti-diabetic drug therapies. The role and effectiveness of micronutrients in various aspects of diabetes have been evaluated in numerous in vitro and in vivo studies. In addition, some natural compounds were subjected to clinical trials. Unfortunately, there are not many clinical and scientific studies evaluating the role of micronutrients in prevention and management of diabetes in children, although several of these natural compounds have been used in managing diabetes in the young.
The requirements for micronutrients vary depending on our age, but also genetics, lifestyle, dietary patterns, various pathologies, and environmental factors. Current official guidelines, such as the Recommended Dietary Allowance (RDA), provide information on quantities of individual nutrients required to maintain health in already healthy individuals. The Adequate Intake (AI) is used when scientific evidence is insufficient to develop an RDA. The Tolerable Upper Intake Level (UL) has been developed to indicate the level of nutrient intake which should pose no adverse health effects in almost all individuals in the general population (National Institutes of Health, NIH). However, these guidelines refer to intake of nutrients as individual compounds and for many people the recommended doses are not adequate to maintain optimum health. Unfortunately, the mutual interactions of micronutrients, their absorption and bioavailability when taken as complexes have not been well investigated. Most vitamins and other micronutrients are taken in forms of random mixtures and with different diets, therefore their final metabolic effects are largely a matter of chance and can’t be effectively controlled. The need to address these and other important aspects in micronutrient supplementation has been advocated by the Dr. Rath Research Institute, whose scientific research has contributed to a better understanding of nutrient interactions and synergy in various aspects of health67.
Metabolic effects of select vitamins in diabetes
Vitamins are natural organic compounds that are not produced by the organism, with a few exceptions, such as vitamins D and K. Vitamin D is produced by the body through the action of the sun, and vitamin K is made in small amounts by gut bacteria. Thus, vitamins must be necessarily obtained in the diet. They have diverse chemical structures and cellular functions and are categorized as water-soluble and fat-soluble vitamins.
Vitamin A
Vitamin A includes fat-soluble retinoids such as retinol, retinal, and retinyl esters. It is found in fruits and vegetables in the form of precursors, known as carotenoids, which are converted in the body into vitamin A according to its needs. Vitamin A is important in supporting immune function, vision process, reproduction, and takes part in cellular communication. Vitamin A-rich sources include liver and fish oils, also milk and eggs. Carotenoids are abundant in leafy green, orange and yellow vegetables, tomato products, fruits and some vegetable oils68.
Based on the fact that diabetes is associated with a compromised antioxidant status in the body, supplementation with antioxidants, such as vitamin A, should play an important role in diabetes. However, most research supporting vitamin A benefits in diabetes derives from in vitro or animal studies, with almost no clinical studies conducted in diabetic patients.
The study using type 2 diabetic BALB/c mice demonstrated a considerable improvement in total antioxidant potential, glycemic control, and a therapeutic effect against pancreatic beta cell degeneration with vitamin A supplementation69. It has also been demonstrated that vitamin A can regulate insulin release70 and is essential for the maintenance of pancreatic β-cell functions71. Dietary vitamin A deprivation itself can induce hyperglycemia and lower insulin secretion in mice, while its supplementation restores glycemic control, normal islet size distribution, and endocrine profiles, among others. The authors of this study suggested that vitamin A is important in people with diabetes71.
Vitamin B12
Vitamin B12 (cobalamin) is a water-soluble vitamin that plays a fundamental role in DNA synthesis, proper red blood cell formation, and neurological functions. It is naturally found in animal products, including fish, meat, poultry, eggs, and milk72.
Several cross-sectional studies and many case reports have documented a widespread deficiency of vitamin B12 in type 2 diabetes patients. In addition, patients with type 1 diabetes-associated pernicious anemia are also frequently deficient in this vitamin73.
A recent study in obese adolescents with pre-diabetes and/or clinical features of insulin resistance showed low or borderline vitamin B12 status74. In addition, treatment with metformin and concomitant use of proton pump inhibitors/histamine H2-antagonists have been associated with higher risk of developing B12 deficiency in patients with type 2 diabetes75.
An earlier investigation determining serum vitamin B12 levels in patients with type 2 diabetes taking metformin for 5 years or longer has shown vitamin B12 deficiency and its borderline deficiency in 8.6% and 26% of patients respectively76. A study by Dekelbab & Kakkannat carried out in 90 children (7-18 years old) showed that obese children are more likely to have vitamin B12 deficiency whether treated with metformin or not77.
Vitamin C
Vitamin C is a water-soluble vitamin and one of the most important micronutrients not produced in the human body. It is an essential cofactor of numerous enzymes and a potent antioxidant. Best sources of vitamin C are fruits and vegetables, including citrus fruits, tomatoes, peppers, kiwifruit, broccoli, strawberries, and Brussels sprouts. It is not naturally present in grains78.
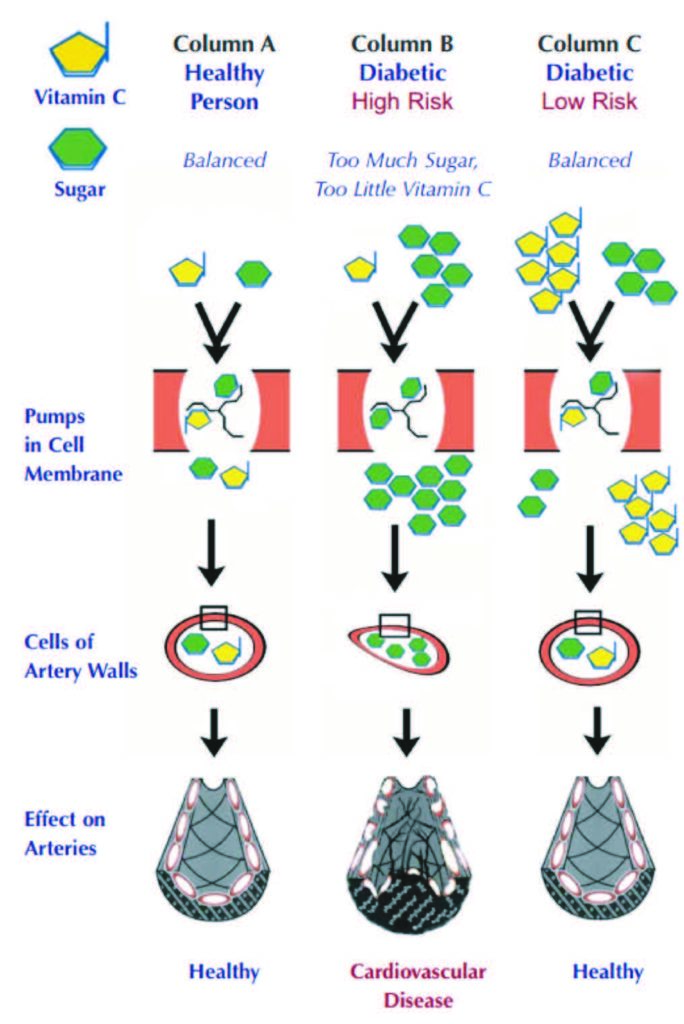
Vitamin C plays a special role in diabetes due to its structural similarity to sugar (glucose) and the use of common intracellular transporters, GLUT1 and GLUT3, by both compounds. As a consequence, elevated glucose levels promote vitamin C deficiency inside the cells by competing with vitamin C for its intracellular entry. This has particularly detrimental consequences for the cardiovascular system in diabetic patients. High glucose related vitamin C deficiency inside the blood vessel wall cells impairs collagen production, thereby compromising vascular integrity and accelerating atherosclerosis as well as microvascular dysfunctions in different organs in diabetic patients (Figure 5)25. Moreover, it has been shown that insufficient ascorbate levels in red blood cells in diabetes promote rigidity of these cells, thereby contributing to microvascular angiopathy79. In addition to impaired intracellular transport, inadequate vitamin C status in diabetic patients is prompted by higher urinary losses of this vitamin and increased metabolic turnover, all contributing to increased dietary requirements for vitamin C in diabetic patients.
Most clinical aspects of vitamin C in diabetes have been evaluated in adults80,81 and confirm compromised vitamin C status, including low vitamin C levels in type 2 diabetic patients with more severe diabetic nephropathy82. A study in 84 patients with type 2 diabetes receiving different doses of vitamin C for six weeks showed that its intake at a level of 1,000 mg a day may be beneficial in decreasing blood glucose and lipid levels, thus reducing the risk of vascular complications83. A recent study with 2,025 children aged 9-10 years investigated the relationship between vitamin C blood levels, fruit and vegetable intakes and insulin resistance. Based on a 24h dietary recall it was found that lower plasma vitamin C levels were associated with insulin resistance in these young individuals84.
Vitamin D
Vitamin D is a fat-soluble vitamin mostly known for facilitating calcium absorption. However this vitamin also affects cell growth, inflammation and various neuromuscular and immune functions. The best sources are fatty fish (salmon, tuna, and mackerel) and fish liver oils. Small amounts of vitamin D are found in beef liver, cheese, and egg yolks85.
The majority of epidemiological studies have demonstrated an association between low vitamin D and insulin resistance and/or type 2 diabetes mellitus. Also, the study in young patients with type 1 diabetes showed that over 95% of them had insufficient (20-30 ng/mL) or deficient (86. The association between vitamin D levels (25(OH)D) and HbA1c was confirmed in 197 diabetic children and adolescents with type 1 diabetes, showing a high prevalence of low vitamin D status in association with type 1 diabetes87. Treatment of 53 pediatric type 1 diabetes patients with vitamin D3 for three months demonstrated better glycemic control and a statistically significant reduction in HbA1C88.
Vitamin D deficiency has been also associated with diabetic peripheral neuropathy89. In the pediatric populationits prevalence ranges from 7% to 54% depending on the diagnostic criteria used, but vitamin D subclinical manifestations occur in about 57% children and adolescents with type 1 diabetes41. In addition, a study of 517 children and adolescents with type 1 diabetes showed that vitamin D deficiency can double the risk of developing retinopathy90.
Vitamin K
Vitamin K is a generic name for a family of quinone compounds such asphylloquinone (vitamin K1) and a series of menaquinones (vitamin K2). Vitamin K functions as a coenzyme for vitamin K-dependent carboxylase, required for the synthesis of proteins involved in hemostasis and bone metabolism and other physiological functions. Best sources of vitamin K1 include vegetables, especially green leafy vegetables, vegetable oils, and some fruits. Meat, dairy foods and eggs contain low levels of vitamin K1, but modest amounts of vitamin K291.
It was reported that vitamin K supplementation has beneficial effects in improving insulin sensitivity92. Both animal and human studies have suggested that a beneficial function of vitamin K in insulin sensitivity and glucose tolerance may be mediated by its regulation of cytokines secreted by adipose tissue, its anti-inflammatory properties and lipid-lowering effects93. In a 3 year randomized, double-blind, placebo controlled trial of 355 patients, vitamin K significantly improved insulin sensitivity in men with diabetes by interfering with pancreatic β-cell proliferation, insulin sensitivity, production of adiponectin, and glucose tolerance94. Vitamin K effects were not evaluated in diabetic pediatric population.
Metabolic effects of minerals in various aspects of diabetes
Chromium
Chromium, required in trace amounts in humans, is known to enhance the action of insulin. Chromium is widely distributed in food, but only in small quantities (less than 2 mcg per serving). Good sources include meat and wholegrain products, some fruits, vegetables, and spices95.
The evaluation of chromium levels in plasma, erythrocytes and urine in 47 children diagnosed with type 1 diabetes and 118 non-diabetic controls showed a negative chromium balance in diabetic subjects, suggesting that chromium supplementation together with insulin may be necessary96. A recent study from Harvard Medical School showed that the odds of having type 2 diabetes were lower in adults who, in the previous 30 days, had consumed supplements containing chromium97. However, chromium supplementation in pediatric diabetic patients has not been evaluated.
Iron
Iron is an essential element in almost all living organisms. It is needed in oxygen transport (as a component of hemoglobin), bioenergy production (electron transport) and normal cellular functions. Dietary iron is available in two main forms: heme and non-heme. Meat, seafood and poultry contain both heme and non-heme iron. Plants such as nuts, beans, vegetables and fortified grain products are the sources of non-heme iron98.
Anemia can occur in diabetic children and may contribute to various complications of the disease, such as cognitive impairment. In 100 children with type 1 diabetes (age 6-17 years), red blood cells, hemoglobin, glycosylated hemoglobin, hematocrit, red blood cell volume, the molar mass of hemoglobin in red blood cells, mean corpuscular hemoglobin in red blood cells, and iron concentrations in serum were measured. The results showed that in the group of children with type 1 diabetes, a significantly lower concentration of three ferric parameters affected non-verbal intelligence. The authors concluded that the prevalence of reduced ferric parameters justifies preventive measures to reduce the risk of anemia in diabetic children99.
Magnesium
Magnesium is an abundant mineral in the body and a cofactor in more than 300 enzyme systems that regulate diverse biochemical reactions, including protein synthesis, muscle and nerve function, blood glucose control, and regulation of blood pressure. It is required for bioenergy production, oxidative phosphorylation, and glycolysis. Magnesium also plays a role in nerve impulse conduction, muscle contraction, and normal heart rhythm. Good sources include green leafy vegetables (i.e. spinach), legumes, nuts, seeds, and whole grains100.
Over the past decades, hypomagnesemia (serum magnesium 101, and also that subjects with poor glycemic control had low magnesium levels102,103,104. Magnesium supplementation or its increased intake can be important in prevention of type 2 diabetes in obese children105.
Selenium
Selenium, a trace element, is a constituent of more than two dozen seleno-proteins that play critical roles in thyroid hormone metabolism, DNA synthesis, reproduction, protection from oxidative damage and infections. Its best sources are seafood and organ meats, others include muscle meats, cereals and other grains and dairy products106.
Evaluation of oxidant status and micronutrient levels in 35 children with type 1 diabetes and 26 healthy children showed that selenium and zinc levels were significantly lower in diabetic subjects than in controls and inversely correlated with HbA1C levels. Diabetic children also had lower levels of glutathione peroxidase, which requires selenium for its activity. This study concluded that supplementation of these minerals can be beneficial in controlling diabetes and preventing its complications107.
Zinc
Zinc is an essential mineral required for the catalytic activity of approximately 100 enzymes involved in immune function, protein synthesis, wound healing, DNA synthesis, and cell division. Zinc also supports normal growth and development during pregnancy, childhood and adolescence and is required for proper sense of taste and smell. Oysters contain more zinc per serving than any other food, but red meat, poultry, beans, nuts, certain types of seafood (such as crab and lobster), whole grains, fortified breakfast cereals, and dairy products are also good sources108.
Zinc is essential for the normal processing and storage of insulin and might enhance glucose cellular intake by modulating insulin signaling pathways109. The first comprehensive systematic review and meta-analysis on the effects of zinc supplementation in patients with diabetes, involving three studies on type 1 diabetes and 22 studies on type 2 diabetes, demonstrated that zinc supplementation has beneficial effects on glycemic control and healthy lipid parameters110.
Reports in the literature on the zinc status of children and adolescents with type 1 diabetes mellitus are limited and contain contradictory results. Some investigators have shown decreased serum zinc concentrations in subjects with diabetes111,112, while in earlier studies its elevated levels were observed as compared to non-diabetic controls113. A few studies observed no changes in zinc status114,115. However, a recent study involving 88 children with type 1 diabetes and 76 healthy controls concluded that while there was no difference in zinc levels between these groups, there was a significant difference between zinc levels and insulin dose/BMI in diabetics101. A study involving 17 children with insulin-dependent diabetes indicated a secondary zinc deficiency in these children that could increase a risk of stunted growth 116. It appears that zinc supplementation is beneficial in controlling diabetes in children.
Metabolic effects of phytobiologicals in diabetes
Phytobiologicals (also called phytochemicals) include a large group of plant-derived compounds, which include phenolic acids, flavonoids, stilbenes/lignanes, and many others117. They are widely present in fruits, vegetables, beans, cereals and plant-based beverages such as tea and wine117. Their higher intake has been associated with many positive effects on diabetes118,119,120,121,122, but the effects of individual phytobiological compounds in children have not been thoroughly evaluated. Most information regarding efficacy of these secondary plant metabolites in the prevention and management of diabetes has been based on laboratory studies or on reports of their intake in adults.
Cinnamon. Cinnamon is a common spice obtained from the inner bark of Cinnamonum trees. Cinnamon extract is an insulin sensitizer, protects mesangial cells, decreases inflammatory markers, and lowers glucose, lipids, and blood pressure in patients with type 2 diabetes123.
The effects of cinnamon intake as an adjunct to a suffonylurea drug (glibenclamide) in 25 adult patients with type 2 diabetes showed that those taking cinnamon had reduced fasting blood glucose levels, less glycosylated HbA1c, and improve doxidative stress markers compared to a placebo124. Also, the efficacy of polyphenol–rich cinnamon extracts in lowering blood glucose levels and ameliorating oxidative stress was demonstrated in 15 volunteers with elevated fasting blood glucose125.
Curcumin. Curcumin, the main component of the Indian spice turmeric, has been used in traditional medicine to improve diabetes and its comorbidities. Its supplementation has been linked to improved insulin resistance through activation of the insulin receptor and related metabolic pathways126. Recently, Weisberg and collaborators showed that dietary intake of curcumin was associated with increased insulin production and prevention of hyperglycemia127. Curcumin binds directly to GLUT1 receptors, thereby inhibiting glucose transport. In intestinal epithelial cells this would likely result in reduced absorption of dietary glucose and a hypoglycemic effect128. Animal studies indicate that curcumin can help in alleviating heart microvascular diabetic complications129. It can benefit in diabetic nephropathy by inhibiting renal lipid accumulation and decreasing oxidative stress through AMPK and Nrf2 signaling pathway130. Its value has also been shown in diabetes induced periodontal disease131. Curcumin supplementation has not been evaluated in children.
Green tea extract. Epigallocatechin gallate (EGCG) is an antioxidant compound present in green tea. Epidemiological studies have demonstrated correlations between green tea consumption and reduced risk of type 2 diabetes and its cardiovascular complications. EGCG can oppose endothelial dysfunction and ameliorate metabolic insulin resistance132. Green tea polyphenols were beneficial in various aspects of diabetes in adults133,134, but have not been investigated in pediatric diabetes.
Quercetin. Quercetin is an antioxidant widely distributed in plants and, together with its glycosides, exerts anti-diabetic properties by interfering with insulin signaling in peripheral target tissues. Quercetin positively affects glucose metabolism in the liver and skeletal muscle135, and is able to reduce blood glucose levels by stimulating insulin release136 and insulin sensitivity137. No studies were done in the pediatric population.
Resveratrol. Resveratrol is a polyphenol whose anti-diabetic potential has been confirmed in several studies. Its benefits include increased cerebral vasodilator responsiveness in the cerebral arteries138, as well as anti-inflammatory and hepato-protective effects139. Resveratrol may also be considered as a therapeutic option to prevent diabetic retinopathy140. No clinical studies are available in the pediatric population.
Coenzyme Q10 and diabetes
Coenzyme Q10 (CoQ10), also known as ubiquinone, is an antioxidant and essential component in the synthesis of adenosine triphosphate (ATP) in the mitochondrial bioenergy cycle. CoQ10 is synthesized internally but the ability to produce this nutrient decreases with age. Fish, meats and whole grains all have small amounts of CoQ10, but not enough to significantly boost its levels in the body141.
There is a growing body of evidence suggesting that mitochondrial dysfunction secondary to oxidative stress plays a critical role in the pathogenesis of type 2 diabetes. It was found that administration of CoQ10 could ameliorate cell apoptosis induced by high glucose and increase mitochondrial membrane potential, as well as reduce oxidative stress. These findings provide a potential treatment strategy targeting dysfunctional endothelial progenitor cells in diabetic patients142.
Menke and colleagues measured CoQ10 concentrations in plasma and blood cells, and redox status, in children with type 1 diabetes and healthy children. The study found that the level of plasma CoQ10 in children with type 1 diabetes was higher compared to that of healthy children. The authors suggested that in children with poorly controlled diabetes, an increase in antioxidant CoQ10 and intracellular redox capacity may represent self-protection of the body during the development of oxidative stress143.
Role of fiber in diabetes
Dietary fiber comprises the edible parts of plants that cannot be digested or absorbed in the small intestine and pass into the large intestine intact. It is often categorized based on its solubility into soluble and insoluble fiber present in different proportions in fiber-containing foods.
Rich sources of soluble fiber include oats, barley, fruits, vegetables and pulses (beans, lentils, chickpeas), while insoluble fiber is found in wholegrain cereals and breads.
Soluble fiber can slow digestion and absorption of carbohydrates and hence lower the rise in blood glucose that follows a meal (postprandial) and insulin response. This can help people with diabetes in controlling their blood glucose levels. In addition, fiber may contribute to weight control by delaying the gastric emptying of ingested foods into the small intestine, thereby creating a sensation of fullness. It can interfere with the absorption of dietary fat and cholesterol, as well as with the entero-hepatic recirculation of cholesterol and bile acids, which may result in reduced blood cholesterol levels144.
The data consistently confirm positive effects of dietary fiber on lowering risk of diabetes and its symptoms in general145. However, there are not many studies evaluating its effect in children. A randomized clinical trial examining longitudinally the association of dietary fiber intake with multiple indicators of glycemic control in youth with type 1 diabetes concluded that glycemic control may be improved by increasing intake of high-fiber, low glycemic-index, carbohydrate-containing foods146. This was in agreement with the results of an earlier study conducted in type 2 diabetic children which showed that psyllium intake decreased postprandial glucose147. Also, a study in Latino children showed that children without symptoms of metabolic syndrome had higher soluble fiber intake148, but others found no association149. The differences may relate to quality and type of fiber intake.
Micronutrient synergy and its application in young organisms with diabetes
Biological effects of micronutrients largely depend on mutual interactions and cooperation with other natural components in numerous complex cellular metabolic pathways. The new approach in dietary supplementation developed at the Dr. Rath Research Institute and introduced as “micronutrient synergy” allows for application of specific combinations of micronutrients in a controlled way. Synergy allows for expanded metabolic efficacy of micronutrients by simultaneously targeting several cellular mechanisms associated with various pathologies and enhanced efficacy with lower doses of individual components. Micronutrient synergy presents a new strategy in developing effective natural approaches against various human pathologies, including diabetes.
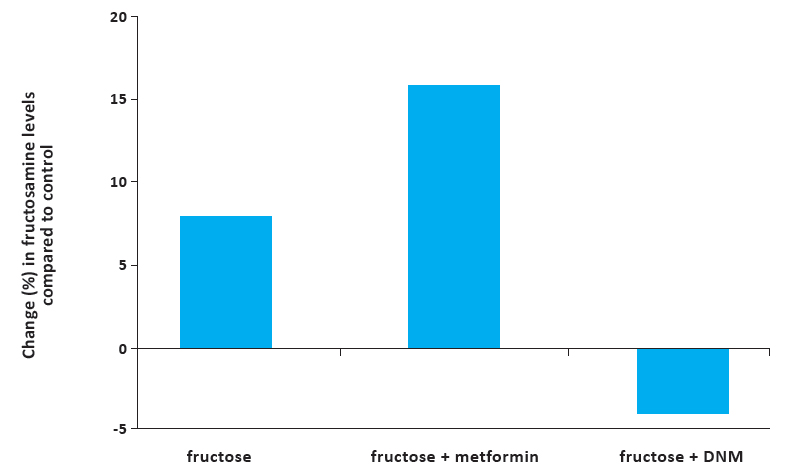
An in vivo study conducted in young mice with diabetes showed that a specific micronutrient synergy was superior to a diabetes drug, metformin, in regulating blood sugar levels and other aspects of diabetes150. Diabetes was induced in these young mice by feeding them a fructose-rich diet, which indicates its relevance to the effects of high fructose consumption in youth and adolescence. Fructose-induced protein damage was evaluated by measuring serum levels of fructosamine, a marker of protein damage in diabetes. The results showed that dietary intake of the synergistic micronutrient complex reduced serum fructosamine by about 4%, while metformin caused its 15.9% increase (Figure 6). In addition, insulin blood levels in mice in the micronutrient supplemented group were restored to normal values, while the metformin group had reduced insulin. In addition, micronutrient supplementation showed to be beneficial in reducing the risk of cardiovascular disease in these animals by lowering blood pressure, total cholesterol, and counteracting other negative effects of fructose150. These results indicate that synergistically interacting micronutrient composition results in more comprehensive anti-diabetic effects than individual compounds.
Figure 6. Comparative effects of metformin and diabetic nutrient mixture on fructosamine levels in fructose-fed mice. The DNM group demonstrated significantly lower fructosamine concentration (3.94% decrease) than the control (fructose-only fed) group, whereas the MET- treated group showed a 15.9% increase in fructosamine compared to the control. A t-test performed between the DNM group vs the fructose alone group and ANOVA for all groups yielded p<0.05150.
Conclusions
Evaluation of research, clinical and therapeutic approaches to diabetes in children and young adults indicates the need for intensifying research efforts to address unique aspects of this disease in this age group. In particular, specific attention should be placed on the benefits of vitamins, minerals, phytochemicals, and other natural compounds as safe and effective measures in prevention and management of diabetes in children. Health efficacy of these micronutrients has already been indicated in various clinical and scientific studies and supported by their practical application in a form of dietary supplementation. The major limiting factor in accepting therapeutic efficacy of natural approaches in diabetes as well as many other health problems is a lack of financial interest in advancing access to natural therapies, which do not generate exuberant profit margins like pharmaceutical approaches. Therefore, governmental support of this research direction is warranted.
Scientific and clinical support for the effectiveness of natural therapies against diabetes will also encourage and intensify further research interest in this globally growing health threat.
References
1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med, 2006, 3(11):e442. www.ncbi.nlm.nih.gov/pubmed/17132052
2. WHO, World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Geneva, World Health Organization, 1999.
3. Atkinson M, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014 Jan 4; 383(9911): 69–82. www.ncbi.nlm.nih.gov/pmc/articles/PMC4380133
4. Barat P. Epidemiology of type 1 diabetes in children. SoinsPediatrPueric. 2016 Jan- Feb;(288):10-2. www.ncbi.nlm.nih.gov/pubmed/26776685
5. Imkampe AK, Gulliford MC. Trends in Type 1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991-2008. Diabet Med. 2011 Jul;28(7):811-4. www.ncbi.nlm.nih.gov/pubmed/21395679
6. Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G; EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009 Jun 13;373(9680):2027-33. www.ncbi.nlm.nih.gov/pubmed/19481249/
7. Pinhas-Hamiel O Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 2005;146:693–700. www.ncbi.nlm.nih.gov/pubmed/15870677
8. Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet 2007;369:1823–1831. www.ncbi.nlm.nih.gov/pubmed/17531891
9. Classen JB, Classen DC. Clustering of cases of type 1 diabetes mellitus occurring 2-4 years after vaccination is consistent with clustering after infections and progression to type 1 diabetes mellitus in autoantibody positive individuals. J PediatrEndocrinolMetab. 2003 Apr- May;16(4):495-508. www.ncbi.nlm.nih.gov/pubmed/12793601
10. Classen JB, Classen DC. Clustering of cases of insulin dependent diabetes (IDDM) occurring three years after hemophilus influenza B (HiB) immunization support causal relationship between immunization and IDDM. Autoimmunity. 2002 Jul;35(4):247-53. www.ncbi.nlm.nih.gov/pubmed/12482192
11. Al-Zubeidi H, Leon-Chi L, Newfield RS. Low vitamin D level in pediatric patients with new onset type 1 diabetes is common, especially if in ketoacidosis. Pediatr Diabetes. 2015 Dec 23. www.ncbi.nlm.nih.gov/pubmed/26694737
12. Liu C et al. Correlation of serum vitamin d level with type 1 diabetes mellitus in children: a meta- analysis. Nutr Hosp. 2015 Oct 1;32(4):1591-4. www.ncbi.nlm.nih.gov/pubmed/26545522
13. WHO, World Health Organization. What are the risk of diabetes in children? www.who.int/features/qa/65/en/
14. Copeland C, Becker D, Gottschalk M, Hale D. Type 2 Diabetes in Children and Adolescents: Risk Factors, Diagnosis, and Treatment. Clinical Diabetes 2005 Oct; 23(4): 181-185. www.clinical.diabetesjournals.org/content/23/4/181
15. Wheelock KM, Sinha M, Knowler WC, Nelson RG, Fufaa GD, Hanson RL. Metabolic Risk Factors and Type 2 Diabetes Incidence in American Indian Children J ClinEndocrinolMetab. 2016 Feb 25:jc20154309. www.ncbi.nlm.nih.gov/pubmed/26913636
16. Boulton J, Hashem KM, Jenner KH, Lloyd- Williams F, Bromley H, Capewell S. How much sugar is hidden in drinks marketed to children? A survey of fruit juices, juice drinks and smoothies. BMJ Open. 2016 Mar 23;6(3):e010330. www.ncbi.nlm.nih.gov/pubmed/27009146
17. Ma Y, He FJ, Yin Y, Hashem KM, MacGregor GA. Gradual reduction of sugar in soft drinks without substitution as a strategy to reduce overweight, obesity, and type 2 diabetes: a modelling study. Lancet Diabetes Endocrinol. 2016 Feb;4(2):105-14. www.ncbi.nlm.nih.gov/pubmed/26777597
18. Johnson RJ, Segal MS, Sautin Y. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J ClinNutr 2007;86(4):899–906. www.ncbi.nlm.nih.gov/pubmed/17921363
19. Segal MS, Gollub E, Johnson RJ. Is the fructose index more relevant with regards to cardiovascular disease than the glycemic index? Eur J Nutr 2007;46(7):406–17. www.ncbi.nlm.nih.gov/pubmed/17763967
20. Nakagawa T et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol 2006;290(3): F625–31. www.ncbi.nlm.nih.gov/pubmed/16234313
21. WHO, Word Health Organization European Region. Data and statistics. www.euro.who.int/en/health-topics/noncommunicable-diseases/obesity/data-and-statistics
22. WHO, World Health Organization. Physical activity. www.who.int/mediacentre/factsheets/fs385/en/
23. Fernandes RA, Zanesco A. Early sport practice is related to lower prevalence of cardiovascular and metabolic outcomes in adults independently of overweight and current physical activity. Medicina (Kaunas). 2015;51(6):336-42. www.ncbi.nlm.nih.gov/pubmed/26739675
24. Huus K, Åkerman L, Raustorp A, Ludvigsson J. Physical Activity, Blood Glucose and C-Peptide in Healthy School-Children, a Longitudinal Study. PLoS One. 2016 Jun 7;11(6):e0156401. www.ncbi.nlm.nih.gov/pubmed/27270732
25. Rath M. Why animals don´t get heart attack but people do! Chapter 7: Diabetes, 2003.
26. Chrysis D, Efthymiadou A, Mermigka A, Kritikou D, Spiliotis BE. Osteoprotegerin, RANKL, ADMA, and Fetuin-A serum levels in children with
type I diabetes mellitus. Pediatr Diabetes. 2016 Mar 29. www.ncbi.nlm.nih.gov/pubmed/27028343
27. Song SH, Hardisty CA. Early-Onset Type 2
Diabetes Mellitus: An Increasing Phenomenon of Elevated Cardiovascular Risk. Expert Rev CardiovascTher. 2008;6(3):315-322. www.medscape.com/viewarticle/573459
28. al-Naama LM, Kadhim M, al-Aboud MS. Lipid profile in children with insulin dependent diabetes mellitus. J Pak Med Assoc. 2002
Jan;52(1):29-34. www.ncbi.nlm.nih.gov/pubmed/11963582
29. Petitti DB et al. Serum lipids and glucose control: the SEARCH for Diabetes in Youth study. Arch PediatrAdolesc Med. 2007 Feb;161(2):159-65. www.ncbi.nlm.nih.gov/pubmed/17283301
30. Reinehr T et al. Inflammatory Markers in Obese Adolescents with Type 2 Diabetes and Their Relationship to Hepatokines and Adipokines.J Pediatr. 2016 Mar 17. www.ncbi.nlm.nih.gov/pubmed/26996723
31. Freemark M, Levitsky L. Screening for Celiac Disease in Children with Type 1 Diabetes. Diabetes Care 2003 Jun; 26(6): 1932-1939. http://care.diabetesjournals.org/content/26/6/1932
32. Kordonouri O, Hartmann R, Deiss D, Wilms M, Gruters-Kieslich A. Natural course of autoimmune thyroiditis in type 1 diabetes: association with gender, age, diabetes duration, and puberty. Arch Dis Child. 2005 Apr; 90(4): 411–414. www.ncbi.nlm.nih.gov/pmc/articles/PMC1720371/
33. Lu MC, Chang SC, Huang KY, Koo M, Lai NS.
Higher Risk of Thyroid Disorders in Young Patients with Type 1 Diabetes: A 12-Year Nationwide, Population-Based, Retrospective Cohort Study. PLoS One. 2016 Mar
23;11(3):e0152168. www.ncbi.nlm.nih.gov/pubmed/27007574
34. Litmanovitch E, Geva R, Rachmiel R. Short and long term neuro-behavioral alterations in type 1 diabetes mellitus pediatric population. World J Diabetes. 2015 Mar 15; 6(2): 259–270. www.ncbi.nlm.nih.gov/pmc/articles/PMC4360419/
35. Jabbour G, Henderson M, Mathieu ME. Barriers to Active Lifestyles in Children with Type 1 Diabetes. Can J Diabetes. 2016 Apr;40(2):170-2. www.ncbi.nlm.nih.gov/pubmed/27038139
36. Brady CC et al. Obese adolescents with type 2 diabetes perform worse than controls on cognitive and behavioral assessments. Pediatr Diabetes. 2016 Mar 29. www.ncbi.nlm.nih.gov/pubmed/27028236
37. Suh JS et al.Urinary markers in the early stage of nephropathy in patients with childhood-onset type 1 diabetes. PediatrNephrol. 2016 31(4):623-31. www.ncbi.nlm.nih.gov/pubmed/26525196
38. Papadopoulou-Marketou N et al.NGAL and cystatin C: two possible early markers of diabetic nephropathy in young patients with type 1 diabetes mellitus: one year follow up. Hormones (Athens). 2015 Apr-Jun;14(2):232-40. www.ncbi.nlm.nih.gov/pubmed/25402375
39. Craig EM, Craig J, DabeleaD,Balde N, Seth A, Donaghue KC. ISPAD Clinical Practice Consensus Guidelines 2014 Compendium. Definition, epidemiology, and classification of diabetes in children and adolescents. http://onlinelibrary.wiley.com/doi/10.1111/pedi.12186/abstract
40. Li L, Jick S, Breitenstein S, Michel A. Prevalence of Diabetes and Diabetic Nephropathy in a Large U.S. Commercially Insured Pediatric Population,
2002–2013. Diabetes Care 2016 Feb; 39(2):278-284. http://care.diabetesjournals.org/content/39/2/278
41. Trotta D, Verrotti A, Salladini C, Chiarelli F.
Diabetic neuropathy in children and adolescents. Pediatr Diabetes. 2004 Mar;5(1):44-57. http://www.ncbi.nlm.nih.gov/pubmed/15043690
42. Hamid A, Wharton HM, Mills A, Gibson JM, Clarke M, Dodson PM. Diagnosis of retinopathy in children younger than 12 years of age: implications for the diabetic eye screening guidelines in the UK. Eye (Lond). 2016 Apr 15. http://www.ncbi.nlm.nih.gov/pubmed/27080488
43. Hermann G et al. Comorbidity of Type 1 Diabetes and Juvenile Idiopathic Arthritis. April 2015 Volume 166, Issue 4, Pages 930–935.e3. http://www.jpeds.com/article/S0022-3476(14)01195-0/fulltext
44. Mantravadi M. Pediatric Osteoporosis, 2016. http://emedicine.medscape.com/article/985221-overview#a3
45. Lalla E et al. Periodontal Changes in Children and Adolescents With Diabetes. Diabetes Care 2006 Feb; 29(2): 295-299. http://care.diabetesjournals.org/content/29/2/295
46. Xavier AC, Silva IN, Costa Fde O, Corrêa DS. Periodontal status in children and adolescents with type 1 diabetes mellitus. Arq Bras EndocrinolMetabol. 2009 Apr;53(3):348-54. http://www.ncbi.nlm.nih.gov/pubmed/19578597
47. American Diabetes Association. Skin Complications. www.diabetes.org/living-with-diabetes/complications/skin-complications
48. Kowalewska B, Kawko M, Zorena K, Myśliwiec M. Yeast-like fungi in the gastrointestinal tract in children and adolescents with diabetes type 1. PediatrEndocrinol Diabetes Metab. 2015;20(4):170-7. http://www.ncbi.nlm.nih.gov/pubmed/26615584
49. Kowalewska B, Zorena K, Szmigiero-Kawko M, Wąż P, Myśliwiec M. Higher diversity in fungal species discriminates children with type 1 diabetes mellitus from healthy control. Patients Prefer Adherence. 2016; 10:591-599. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4844445/
50. FDA, Food and Drug administration. Code of Federal Regulation. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=310.517
51. Onge ES, Miller SA, Motycka C, DeBerry A. A review of the treatment of type 2 diabetes in children. J PediatrPharmacolTher. 2015 Jan-Feb;20(1):4-16. http://www.ncbi.nlm.nih.gov/pubmed/25859165
52. Cherubini V, Pintaudi B, Iannilli A, Pambianchi M, Ferrito L, Nicolucci A. Long-acting Insulin Analogs Effect on gh/igf Axis of Children with Type 1 Diabetes: a Randomized, Open-label, Two-period, Cross-over Trial. ExpClinEndocrinol Diabetes. 2016 Mar 29. http://www.ncbi.nlm.nih.gov/pubmed/27023008
53. Cope JU, Samuels-Reid JH, Morrison AE. Pediatric Use of Insulin Pump Technology: A Retrospective Study of Adverse Events in Children Ages 1–12 Years J Diabetes Sci Technol.2012 Sep; 6(5): 1053–1059. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3570839/
54. Gao YQ , Gao M, Xue Y. Treatment of diabetes in children. ExpTher Med. 2016 Apr;11(4):1168-1172 http://www.ncbi.nlm.nih.gov/pubmed/27073417
55. Libman IM et al. Effect of Metformin Added to Insulin on Glycemic Control Among Overweight/ Obese Adolescents With Type 1 Diabetes: A Randomized Clinical Trial. JAMA. 2015 Dec 1;314(21):2241-50. http://www.ncbi.nlm.nih.gov/pubmed/26624824
56. Lysy J, Israeli E, Goldin E. The prevalence of chronic diarrhea among diabetic patients. Am J Gastroenterol. 1999 Aug;94(8):2165-70.
57. Pozzo AM. Pediatric Type 2 Diabetes Mellitus Medication. Medscape, May 2014. http://reference.medscape.com/article/925700-medication
58. Wen YW, Tsai YW, Huang WF, Hsiao FY, Chen PF.The potentially inappropriate prescription of new drug: thiazolidinediones for patients with type II diabetes in Taiwan. Pharmacoepidemiol Drug Saf. 2011 Jan;20(1):20-9. http://www.ncbi.nlm.nih.gov/pubmed/21182151
59. Ahuja V, Chou CH. Novel Therapeutics for Diabetes: Uptake, Usage Trends, and Comparative Effectiveness. CurrDiab Rep. 2016 Jun;16(6):47. http://www.ncbi.nlm.nih.gov/pubmed/27076180
60. Onge ES, Miller SA, Motycka C, DeBerry A. A review of the treatment of type 2 diabetes in children. J PediatrPharmacolTher. 2015 Jan-Feb;20(1):4-16. http://www.ncbi.nlm.nih.gov/pubmed/25859165
61. FDA, Food and Drug administration. Code of Federal Regulation. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=310.517
62. Yaffe S. Rational Therapeutics for Infants and Children: Workshop Summary. Similarities and Dissimilarities in Physiology, Metabolism, and Disease States and Responses to Therapy in Children and Adults. National Academies Press (US); 2000.
63. Hines RN. Ontogeny of human hepatic cytochromes P450. J BiochemMolToxicol 21:169–175, 2007. http://onlinelibrary.wiley.com/doi/10.1002/jbt.20179/abstract
64. Sahota PS, Popp JA, Hardisty JF, Gopinath G. Toxicologic pathology non clinical safety assessment. Exocrine pancreas. 2013 CRC Press, Taylor & Francis Group.
65. Ulrich AB, Standop J, Schmied BM, Schneider MB, Lawson TA, Pour PM. Species Differences in the Distribution of Drug-Metabolizing Enzymes in the Pancreas. Toxicologic Pathology, vol 30, no 2, pp 247–253, 2002. http://tpx.sagepub.com/content/30/2/247
66. WHO, World Health Organization.Micronutrients.
http://www.who.int/nutrition/topics/micronutrients/en/
67. Dr. Rath Research Institute www.drrathresearch.org
68. NIH, National Institutes of Health. Vitamin A Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/
69. Meerza D, Iqbal S, Zaheer S, Naseem I. Retinoids have therapeutic action in type 2 diabetes. Nutrition 2016 Jul-Aug;32(7-8):898-903. http://www.ncbi.nlm.nih.gov/pubmed/27134203
70. Iqbal S, Naseem I. Role of vitamin A in type 2 diabetes mellitus biology: Effects of intervention therapy in a deficient state. Nutrition. July– August, 2015 Volume 31, Issues 7-8, Pages 901–907. http://www.nutritionjrnl.com/article/S0899-9007(14)00552-8/fulltext
71. Trasino SE, Benoit DY, Gudas LJ. Vitamin A Deficiency Causes Hyperglycemia and Loss of Pancreatic β-Cell Mass. J BiolChemistry. 2015, 290(3), 1456-1473. http://www.ncbi.nlm.nih.gov/pubmed/25451926
72. NIH, National Institutes of Health. Vitamin B12 Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/
73. Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J. DiabMetab Disorders 2013; 12:7. https://jdmdonline.biomedcentral.com/articles/10.1186/2251-6581-12-17
74. Ho M et al. Vitamin B12 in obese adolescents with clinical features of insulin resistance. Nutrients. 2014 Dec 4;6(12):5611-8. https://www.ncbi.nlm.nih.gov/pubmed/25486369
75. Damião CP et al. Prevalence of vitamin B12 deficiency in type 2 diabetic patients using metformin: a cross-sectional study. Sao Paulo Med J. 2016 Jun 3. http://www.ncbi.nlm.nih.gov/pubmed/27276084
76. Akinlade KS, Agbebaku SO, Rahamon SK, Balogun WO. Vitamin B12 levels in patients with type 2 diabetes mellitus on metformin. Ann IbPostgrad Med. 2015 Dec;13(2):79-83. http://www.ncbi.nlm.nih.gov/pubmed/27162518
77. Dekelbab B, Kakkannat A. Prevalence of Vitamin B12 Deficiency in Children and Adolescents Treated with Metformin. Endocrine Society’s
96th Annual Meeting and Expo, June 21–24, 2014 – Chicago.
78. NIH, National Institutes of Health. Vitamin C Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/#h2
79. Tu H et al. Low Red Blood Cell Vitamin C Concentrations Induce Red Blood Cell Fragility: A Link to Diabetes Via Glucose, Glucose Transporters, and Dehydroascorbic Acid. EBioMedicine. 2015 Oct 3;2(11):1735-50. http://www.ncbi.nlm.nih.gov/pubmed/26870799
80. Li L, Jick S, Breitenstein S, Michel A. Prevalence of Diabetes and Diabetic Nephropathy in a Large U.S. Commercially Insured Pediatric Population,
2002–2013. Diabetes Care 2016 Feb; 39(2): 278-284. http://care.diabetesjournals.org/content/39/2/278
81. Christie-David DJ, Girgis CM, Gunton JE.
Effects of vitamin C and D in type 2 diabetes mellitus, Nutr and Dietary Suppl 2015, 15(7), 21-28. www.researchgate.net/publication/272092706_Effects_of_vitamins_C_and_D_in_type_2_diabetes_mellitus
82. Chou ST, Tseng ST. Oxidative stress markers in type 2 diabetes patients with diabetic nephropathy. ClinExpNephrol. 2016 May 27. http://www.ncbi.nlm.nih.gov/pubmed/27233502
83. ArdekaniMA ,Ardekani AS. Effect of vitamin C on blood glucose, serum lipids & serum insulin in type II diabetes patients. Indian J Med Research, vol. 126, no. 5, pp. 471–474, 2007. http://www.ncbi.nlm.nih.gov/pubmed/18160753
84. Donin AS et al. Fruit, vegetable and vitamin C intakes and plasma vitamin C: cross-sectional associations with insulin resistance and glycaemia in 9-10 year-old children. Diabet Med. 2016 Mar;33(3):307-15. http://www.ncbi.nlm.nih.gov/pubmed/26498636
85. NIH, National Institutes of Health. Vitamin D Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
86. Mosso C, Hodgson MI, Ortiz T, Reyes ML.
Bone mineral density in young Chilean patients with type 1 diabetes mellitus. J PediatrEndocrinolMetab. 2016 Jun 1;29(6):731-6. http://www.ncbi.nlm.nih.gov/pubmed/27054593
87. Al Sawah S, Compher CW, Hanlon AL, Lipman TH. 25-Hydroxyvitamin D and glycemic control: A cross-sectional study of children and adolescents with type 1 diabetes. Diabetes Res ClinPract. 2016 May;115:54-9. http://www.ncbi.nlm.nih.gov/pubmed/27242123
88. Mohammadian S, Fatahi N, Zaeri H, Ali Vakili M. Effect of Vitamin D3 supplement in glycemic control of pediatrics with type 1 diabetes mellitus and vitamin D deficiency.J Clin Diagn Res 2015, 9(3), SC05-SC07. https://www.ncbi.nlm.nih.gov/pubmed/25954674
89. He R et al. Vitamin D deficiency increases the risk of peripheral neuropathy in Chinese patients with Type 2 diabetes. Diabetes Metab Res Rev. 2016 May 7. http://www.ncbi.nlm.nih.gov/pubmed/27155442
90. Kaur H et al. Vitamin D deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. Diabetes Care. 2011 Jun;34(6):1400-2. https://www.ncbi.nlm.nih.gov/pubmed/21515836
91. NIH, National Institutes of Health. Vitamin K Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminK-HealthProfessional/#h3
92. Choi HJ et al. Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: a placebo-controlled trial. Diabetes Care. 2011 Sep;34(9):e147. https://www.ncbi.nlm.nih.gov/pubmed/21868771
93. Manna P, Kalita J. Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: A review. Nutrition. 2016 Jul-Aug;32(7-8):732-9. http://www.ncbi.nlm.nih.gov/pubmed/27133809
94. DiNicolantonio JJ, Bhutani J, O’Keefe JH. The health benefits of vitamin K. Open Heart. 2015 Oct 6;2(1):e000300. http://www.ncbi.nlm.nih.gov/pubmed/26468402
95. NIH, National Institutes of Health. Chromium Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Chromium-HealthProfessional/
96. Karagun BS et al.Chromium levels in healthy and newly diagnosed type 1 diabetic children. Pediatr Int. 2012 54(6):780-785. https://www.ncbi.nlm.nih.gov/pubmed/22783884
97. McIver DJ, Grizales AM, Brownstein JS, Goldfine AB. Risk of Type 2 Diabetes Is Lower in US Adults Taking Chromium-Containing Supplements. J Nutr. 2015 Dec;145(12):2675-82. http://www.ncbi.nlm.nih.gov/pubmed/26446484
98. NIH, National Institutes of Health. Iron Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
99. Mojs E, Stanisławska-Kubiak M, Wójciak RW, Wojciechowska J, Przewoźniak S. Reduced iron parameters and cognitive processes in children and adolescents with DM1 compared to those with standard parameters. J Investig Med. 2016 Mar;64(3):782-5. http://www.ncbi.nlm.nih.gov/pubmed/26912011
100. NIH, National Institutes of Health. Magnesium Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
101. Lin CC, Tsweng GJ, Lee CF, Chen BH, Huang YL. Magnesium, zinc, and chromium levels in children, adolescents, and young adults with type 1 diabetes. Clin. Nutr2016; 35, 880-884. https://www.ncbi.nlm.nih.gov/pubmed/26096861
102. Galli-Tsinopoulou A et al. Association between magnesium concentration and HbA1c in children and adolescents with type 1 diabetes mellitus. J Diabetes, 2014; 6(4), 369-377. https://www.ncbi.nlm.nih.gov/pubmed/24393429
103. Matthiesen G, Olofsson K, Rudnicki M. Ionized magnesium in Danish children with type 1 diabetes. Diabetes Care 2004, 27(3), 1216-1217. http://care.diabetesjournals.org/content/27/5/1216
104. Uğurlu V, Binay Ç, Şimşek E, Bal C. Cellular Trace Element Changes in Type 1 Diabetes Patients. J Clin Res PediatrEndocrinol. 2016 Jun 5;8(2):180-6. http://www.ncbi.nlm.nih.gov/pubmed/27086726
105. Huerta MG et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care 2005; 28(5), 1175-1181. http://care.diabetesjournals.org/content/28/5/1175
106. NIH, National Institutes of Health. Selenium Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
107. Özenç S et al. Selenium, zinc and copper levels and their relation with HbA1c status in children with type 1 diabetes mellitus. In t J Diabetes DevCtrs, 2015, 35(4) 514-518. http://link.springer.com/article/10.1007/s13410-015-0327-y
108. NIH, National Institutes of Health. Zinc Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/
109. Wu Y et al. Zinc stimulates glucose consumption by modulating the insulin signaling pathway in L6 myotubes: essential roles of Akt-GLUT4, GSK3β and mTOR-S6K1. J NutrBiochem. 2016 May 31;34:126-135. http://www.ncbi.nlm.nih.gov/pubmed/27295130
110. Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi GR, Katulanda P. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. DiabetolMetab Syndrome 2012; 4:13. https://www.ncbi.nlm.nih.gov/pubmed/22515411
111. Bideci A, Amurdan MO, Cinaz P, Dursun H, Demirel F. Serum zinc, insulin-like growth factor-I and insulin-like growth factor binding protein-3 levels in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18(10):1007–11. https://www.ncbi.nlm.nih.gov/pubmed/16355814
112. Angelova M, Schentov B, Nedkova V, Nikoloff G, Alexiev AI, Petrova Ch. Serum zinc in children with enterocolitis, chronic diarrhea with malabsorption syndrome and type 1 diabetes. Trakia Journal of Sciences. 2006;4:11–7
113. Tuvemo T, Ewald U, Kobbah M, Proos LA. Serum magnesium and protein concentrations during the first five years of insulin-dependent diabetes in children. Acta Paediatr. 1997;418(Suppl):7–10. https://www.ncbi.nlm.nih.gov/pubmed/9055931
114. Rohn RD, Pleban P, Jenkins LL. Magnesium, zinc and copper in plasma and blood cellular components in children with IDDM. Clin Chim Acta. 1993;215(1):21–8. https://www.ncbi.nlm.nih.gov/pubmed/8513565
115. Estakhri M et al.Serum Zinc Levels in Children and Adolescents with Type-1 Diabetes Mellitus. Iran J Public Health. 2011; 40(4): 83–88. https://www.ncbi.nlm.nih.gov/pubmed/23113106
116. Nakamura T, Higashi A, Nishiyama S, Fujimoto S Matsuda. Kinetics of Zinc Status in Children with IDDM. Diabetes Care 1991; 14(7):
553-557. https://www.ncbi.nlm.nih.gov/pubmed/1914794
117. Heneman K, Zidenberg-Cherr S. Some facts about phytochemicals. Center for Health and Nutrition Research University of California. October 2008. http://nutrition.ucdavis.edu/content/infosheets/fact-pro-phytochemical.pdf
118. Jacques PF, Cassidy A, Rogers G, Peterson JJ, Meigs JB, Dwyer JT. Higher Dietary Flavonol Intake Is Associated with Lower Incidence of Type 2 Diabetes1,2. J Nutr. 2013 Sep; 143(9): 1474–1480. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3743276/
119. Jiao H, Hu G, Gu D, Ni X. Having a promising efficacy on type II diabetes, it’s definitely a green tea time. Curr Med Chem. 2015;22(1):70-9. https://www.ncbi.nlm.nih.gov/pubmed/25139278
120. Szkudelski T, Szkudelska K.Anti-diabetic effects of resveratrol. Ann N Y Acad Sci. 2011 Jan;1215:34-9. https://www.ncbi.nlm.nih.gov/pubmed/21261639
121. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp BiochemPhysiol C ToxicolPharmacol. 2003 Jul;135C(3):357-64. https://www.ncbi.nlm.nih.gov/pubmed/12927910
122. Nabavi SF et al. Curcumin: a natural product for diabetes and its complications. CurrTop Med Chem. 2015;15(23):2445-55. https://www.ncbi.nlm.nih.gov/pubmed/26088351
123. Crawford P et al. Assessment of the effect of lifestyle intervention plus water-soluble cinnamon extract on lowering blood glucose in pre-diabetics, a randomized, double-blind, multicenter, placebo controlled trial: study protocol for a randomized controlled trial. Trials. 2016 Jan 5;17:9. http://www.ncbi.nlm.nih.gov/pubmed/26732017
124. Sahib AS. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: A randomized, placebo-controlled clinical trial. J IntercultEthnopharmacol. 2016 Feb 21;5(2):108-13. http://www.ncbi.nlm.nih.gov/pubmed/27104030
125. Im K, Issac A, NM J, Ninan E, Maliakel B, Kuttan R.Effects of the polyphenol content on the anti-diabetic activity of Cinnamomum zeylanicum extracts. Food Funct. 2014 Sep;5(9):2208-20. http://www.ncbi.nlm.nih.gov/pubmed/25051315
126. Jiménez-Osorio AS, Monroy A, Alavez S. Curcumin and insulin resistance-Molecular targets and clinical evidences. Biofactors. 2016 Jun 21. http://www.ncbi.nlm.nih.gov/pubmed/27325504
127. Weisberg S, Leibel R, Tortoriello DV. Proteasome inhibitors, including curcumin, improve pancreatic β-cell function and insulin sensitivity in diabetic mice. Nutr Diabetes. 2016 Apr 25;6:e205. doi: 10.1038/nutd.2016.13. http://www.ncbi.nlm.nih.gov/pubmed/27110686
128. Gunnink LK et al. Curcumin directly inhibits the transport activity of GLUT1. Biochimie. 2016 Jun;125:179-85. http://www.ncbi.nlm.nih.gov/pubmed/27039889
129. Anupunpisit V, Petpiboolthai H, Khimmaktong W. Microvasculature Improvement of Heart in Diabetic Rat with Curcumin Supplementation. J Med Assoc Thai. 2015 Nov;98Suppl 10:S74-83. http://www.ncbi.nlm.nih.gov/pubmed/27276836
130. Kim BH et al. Protective Effects of Curcumin on Renal Oxidative Stress and Lipid Metabolism in a Rat Model of Type 2 Diabetic Nephropathy. Yonsei Med J. 2016 May;57(3):664-73. http://www.ncbi.nlm.nih.gov/pubmed/26996567
131. Elburki MS et al. A novel chemically modified curcumin reduces inflammation-mediated connective tissue breakdown in a rat model of diabetes: periodontal and systemic effects. J Periodontal Res. 2016 Apr 1. http://www.ncbi.nlm.nih.gov/pubmed/27038334
132. Keske MA et al. Vascular and metabolic actions of the green tea polyphenol epigallocatechingallate. Curr Med Chem. 2015;22(1):59-69. http://www.ncbi.nlm.nih.gov/pubmed/25312214
133. Borges CM, Papadimitriou A, Duarte DA, Lopes de Faria JM, Lopes de Faria JB. The use of green tea polyphenols for treating residual
albuminuria in diabetic nephropathy: A double- blind randomised clinical trial. Sci Rep. 2016 Jun 20;6:28282. http://www.ncbi.nlm.nih.gov/pubmed/27320846
134. Raposo D, Morgado C, Pereira-Terra P, Tavares I.
Nociceptive spinal cord neurons of laminae I-III exhibit oxidative stress damage during diabetic neuropathy which is prevented by early antioxidant treatment with epigallocatechin- gallate (EGCG). Brain Res Bull. 2015 Jan;110:68-75. http://www.ncbi.nlm.nih.gov/pubmed/25522867
135. Eid HM, Nachar A, Thong F, Sweeney G, Haddad
PS.The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn Mag. 2015 Jan-Mar;11(41):74-81. http://www.ncbi.nlm.nih.gov/pubmed/25709214
136. Kitti M et al. Quercetin Stimulates Insulin Secretion and Reduces the Viability of Rat INS-1 Beta-Cells. Cell PhysiolBiochem. 2016 Jun 24;39(1):278-293. http://www.ncbi.nlm.nih.gov/pubmed/27336168
137. Yan SX, Li X, Sun CD, Chen KS. Hypoglycemic and hypolipidemic effects of quercetin and its glycosides ZhongguoZhong Yao ZaZhi. 2015 Dec;40(23):4560-7. http://www.ncbi.nlm.nih.gov/pubmed/27141664
138. Wong RH, Nealon RS, Scholey A, Howe PR. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. NutrMetabCardiovasc Dis. 2016 May;26(5):393-9. http://www.ncbi.nlm.nih.gov/pubmed/27105868
139. Pektaş MB et al. Resveratrol Ameliorates the
Components of Hepatic Inflammation and Apoptosis in a Rat Model of Streptozotocin-Induced Diabetes. Drug Dev Res. 2016 Feb;77(1):12-9. http://www.ncbi.nlm.nih.gov/pubmed/26748675
140. Zeng K et al. Resveratrol Prevents Retinal Dysfunction by Regulating Glutamate Transporters, Glutamine Synthetase Expression and Activity in Diabetic Retina. Neurochem Res. 2016 May;41(5):1050-64. http://www.ncbi.nlm.nih.gov/pubmed/26677078
141. NIH, National Institutes of Health. Coenzyme Q10 in depth. https://nccih.nih.gov/health/supplements/coq10
142. Tsai HY et al. Coenzyme Q10 Attenuates High
Glucose-Induced Endothelial Progenitor Cell Dysfunction through AMP-Activated Protein Kinase Pathways. Diabetes Res. 2016;2016:6384759. http://www.ncbi.nlm.nih.gov/pubmed/26682233
143. Menke T, Niklowitz P, Wiesel T, Andler W. Antioxidant level and redox status of coenzyme Q10 in the plasma and blood cells of children with diabetes mellitus type 1.Pediatr Diabetes. 2008; 9(6):540-5. https://www.ncbi.nlm.nih.gov/pubmed/18694454
144. USDA. U.S. Department of Agriculture. Dietary, functional and total fiber. 339-441. https://fnic.nal.usda.gov/sites/fnic.nal.usda.gov/files/uploads/339-421.pdf
145. Velázquez-López L et al. Fiber in Diet Is Associated with Improvement of Glycated Hemoglobin and Lipid Profile in Mexican Patients with Type 2 Diabetes.J Diabetes Res.2016;2016:2980406. http://www.ncbi.nlm.nih.gov/pubmed/27144178
146. Nansel TR, Lipsky LM, Liu A. Greater diet quality is associated with more optimal glycemic control in a longitudinal study of youth with type 1 diabetes. Am J ClinNutr. 2016 Jul;104(1):81-7. http://www.ncbi.nlm.nih.gov/pubmed/27194309
147. Moreno LA et al. Psyllium fibre and the metabolic control of obese children and adolescents. J PhysiolBiochem. 2003;59:235–242. https://www.ncbi.nlm.nih.gov/pubmed/15000455
148. Ventura EE et al. Dietary intake and the metabolic syndrome in overweight Latino children. J Am Diet Assoc 2008, 108, 1355-1359. https://www.ncbi.nlm.nih.gov/pubmed/18656576
149. McKeown NM, Meigis JB, Liu S, Satzman E, Wilson PW, Jacques PF. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 2004; 27, 538-546. https://www.ncbi.nlm.nih.gov/pubmed/14747241
150. Cha JC, Ivanov V, Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M. Nutritional improvement of metabolic syndrome parameters in immature fructose-fed wild-type mice. Mol Med Rep. 2011 Nov- Dec;4(6):1053-9. http://www.ncbi.nlm.nih.gov/pubmed/21874237
Figure 1. Incidence of type 1 diabetes in children aged 0-14 years, by geographical region and over time3
Figure 2. Overweight prevalence among U.S. children and adolescents14
Figure 3. Short and long term behavioral and cognitive reported alterations following type 1 diabetes mellitus onset in children and adolescents according to type 1 diabetes mellitus duration34
Figure 4. Comparison of Candida albicans strains susceptible, intermediate, and resistant to the six tested antifungal drugs in children with type 1 diabetes mellitus (A) and healthy control subjects (B)49
Table 1. Summary of FDA insulin pump adverse event reports for ages 1-12 years, January 1, 1996, through December 31, 200953
Figure 5. Vitamin C Supplementation is an essential measure for diabetic patients in preventing cardiovascular disease25
Figure 6. Comparative effects of metformin and diabetic nutrient mixture on fructosamine levels in fructose-fed mice. The DNM group demonstrated significantly lower fructosamine concentration (3.94% decrease) than the control (fructose-only fed) group, whereas the MET- treated group showed a 15.9% increase in fructosamine compared to the control. A t-test performed between the DNM group vs the fructose alone group and ANOVA for all groups yielded p<0.05150
1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med, 2006, 3(11):e442. www.ncbi.nlm.nih.gov/pubmed/17132052
2. WHO, World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Geneva, World Health Organization, 1999.
3. Atkinson M, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014 Jan 4; 383(9911): 69–82. www.ncbi.nlm.nih.gov/pmc/articles/PMC4380133
4. Barat P. Epidemiology of type 1 diabetes in children. SoinsPediatrPueric. 2016 Jan- Feb;(288):10-2. www.ncbi.nlm.nih.gov/pubmed/26776685
5. Imkampe AK, Gulliford MC. Trends in Type 1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991-2008. Diabet Med. 2011 Jul;28(7):811-4. www.ncbi.nlm.nih.gov/pubmed/21395679
6. Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G; EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009 Jun 13;373(9680):2027-33. www.ncbi.nlm.nih.gov/pubmed/19481249/
7. Pinhas-Hamiel O Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 2005;146:693–700. www.ncbi.nlm.nih.gov/pubmed/15870677
8. Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet 2007;369:1823–1831. www.ncbi.nlm.nih.gov/pubmed/17531891
9. Classen JB, Classen DC. Clustering of cases of type 1 diabetes mellitus occurring 2-4 years after vaccination is consistent with clustering after infections and progression to type 1 diabetes mellitus in autoantibody positive individuals. J PediatrEndocrinolMetab. 2003 Apr- May;16(4):495-508. www.ncbi.nlm.nih.gov/pubmed/12793601
10. Classen JB, Classen DC. Clustering of cases of insulin dependent diabetes (IDDM) occurring three years after hemophilus influenza B (HiB) immunization support causal relationship between immunization and IDDM. Autoimmunity. 2002 Jul;35(4):247-53. www.ncbi.nlm.nih.gov/pubmed/12482192
11. Al-Zubeidi H, Leon-Chi L, Newfield RS. Low vitamin D level in pediatric patients with new onset type 1 diabetes is common, especially if in ketoacidosis. Pediatr Diabetes. 2015 Dec 23. www.ncbi.nlm.nih.gov/pubmed/26694737
12. Liu C et al. Correlation of serum vitamin d level with type 1 diabetes mellitus in children: a meta- analysis. Nutr Hosp. 2015 Oct 1;32(4):1591-4. www.ncbi.nlm.nih.gov/pubmed/26545522
13. WHO, World Health Organization. What are the risk of diabetes in children? www.who.int/features/qa/65/en/
14. Copeland C, Becker D, Gottschalk M, Hale D. Type 2 Diabetes in Children and Adolescents: Risk Factors, Diagnosis, and Treatment. Clinical Diabetes 2005 Oct; 23(4): 181-185. www.clinical.diabetesjournals.org/content/23/4/181
15. Wheelock KM, Sinha M, Knowler WC, Nelson RG, Fufaa GD, Hanson RL. Metabolic Risk Factors and Type 2 Diabetes Incidence in American Indian Children J ClinEndocrinolMetab. 2016 Feb 25:jc20154309. www.ncbi.nlm.nih.gov/pubmed/26913636
16. Boulton J, Hashem KM, Jenner KH, Lloyd- Williams F, Bromley H, Capewell S. How much sugar is hidden in drinks marketed to children? A survey of fruit juices, juice drinks and smoothies. BMJ Open. 2016 Mar 23;6(3):e010330. www.ncbi.nlm.nih.gov/pubmed/27009146
17. Ma Y, He FJ, Yin Y, Hashem KM, MacGregor GA. Gradual reduction of sugar in soft drinks without substitution as a strategy to reduce overweight, obesity, and type 2 diabetes: a modelling study. Lancet Diabetes Endocrinol. 2016 Feb;4(2):105-14. www.ncbi.nlm.nih.gov/pubmed/26777597
18. Johnson RJ, Segal MS, Sautin Y. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J ClinNutr 2007;86(4):899–906. www.ncbi.nlm.nih.gov/pubmed/17921363
19. Segal MS, Gollub E, Johnson RJ. Is the fructose index more relevant with regards to cardiovascular disease than the glycemic index? Eur J Nutr 2007;46(7):406–17. www.ncbi.nlm.nih.gov/pubmed/17763967
20. Nakagawa T et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol 2006;290(3): F625–31. www.ncbi.nlm.nih.gov/pubmed/16234313
21. WHO, Word Health Organization European Region. Data and statistics. www.euro.who.int/en/health-topics/noncommunicable-diseases/obesity/data-and-statistics
22. WHO, World Health Organization. Physical activity. www.who.int/mediacentre/factsheets/fs385/en/
23. Fernandes RA, Zanesco A. Early sport practice is related to lower prevalence of cardiovascular and metabolic outcomes in adults independently of overweight and current physical activity. Medicina (Kaunas). 2015;51(6):336-42. www.ncbi.nlm.nih.gov/pubmed/26739675
24. Huus K, Åkerman L, Raustorp A, Ludvigsson J. Physical Activity, Blood Glucose and C-Peptide in Healthy School-Children, a Longitudinal Study. PLoS One. 2016 Jun 7;11(6):e0156401. www.ncbi.nlm.nih.gov/pubmed/27270732
25. Rath M. Why animals don´t get heart attack but people do! Chapter 7: Diabetes, 2003.
26. Chrysis D, Efthymiadou A, Mermigka A, Kritikou D, Spiliotis BE. Osteoprotegerin, RANKL, ADMA, and Fetuin-A serum levels in children with
type I diabetes mellitus. Pediatr Diabetes. 2016 Mar 29. www.ncbi.nlm.nih.gov/pubmed/27028343
27. Song SH, Hardisty CA. Early-Onset Type 2
Diabetes Mellitus: An Increasing Phenomenon of Elevated Cardiovascular Risk. Expert Rev CardiovascTher. 2008;6(3):315-322. www.medscape.com/viewarticle/573459
28. al-Naama LM, Kadhim M, al-Aboud MS. Lipid profile in children with insulin dependent diabetes mellitus. J Pak Med Assoc. 2002
Jan;52(1):29-34. www.ncbi.nlm.nih.gov/pubmed/11963582
29. Petitti DB et al. Serum lipids and glucose control: the SEARCH for Diabetes in Youth study. Arch PediatrAdolesc Med. 2007 Feb;161(2):159-65. www.ncbi.nlm.nih.gov/pubmed/17283301
30. Reinehr T et al. Inflammatory Markers in Obese Adolescents with Type 2 Diabetes and Their Relationship to Hepatokines and Adipokines.J Pediatr. 2016 Mar 17. www.ncbi.nlm.nih.gov/pubmed/26996723
31. Freemark M, Levitsky L. Screening for Celiac Disease in Children with Type 1 Diabetes. Diabetes Care 2003 Jun; 26(6): 1932-1939. http://care.diabetesjournals.org/content/26/6/1932
32. Kordonouri O, Hartmann R, Deiss D, Wilms M, Gruters-Kieslich A. Natural course of autoimmune thyroiditis in type 1 diabetes: association with gender, age, diabetes duration, and puberty. Arch Dis Child. 2005 Apr; 90(4): 411–414. www.ncbi.nlm.nih.gov/pmc/articles/PMC1720371/
33. Lu MC, Chang SC, Huang KY, Koo M, Lai NS.
Higher Risk of Thyroid Disorders in Young Patients with Type 1 Diabetes: A 12-Year Nationwide, Population-Based, Retrospective Cohort Study. PLoS One. 2016 Mar
23;11(3):e0152168. www.ncbi.nlm.nih.gov/pubmed/27007574
34. Litmanovitch E, Geva R, Rachmiel R. Short and long term neuro-behavioral alterations in type 1 diabetes mellitus pediatric population. World J Diabetes. 2015 Mar 15; 6(2): 259–270. www.ncbi.nlm.nih.gov/pmc/articles/PMC4360419/
35. Jabbour G, Henderson M, Mathieu ME. Barriers to Active Lifestyles in Children with Type 1 Diabetes. Can J Diabetes. 2016 Apr;40(2):170-2. www.ncbi.nlm.nih.gov/pubmed/27038139
36. Brady CC et al. Obese adolescents with type 2 diabetes perform worse than controls on cognitive and behavioral assessments. Pediatr Diabetes. 2016 Mar 29. www.ncbi.nlm.nih.gov/pubmed/27028236
37. Suh JS et al.Urinary markers in the early stage of nephropathy in patients with childhood-onset type 1 diabetes. PediatrNephrol. 2016 31(4):623-31. www.ncbi.nlm.nih.gov/pubmed/26525196
38. Papadopoulou-Marketou N et al.NGAL and cystatin C: two possible early markers of diabetic nephropathy in young patients with type 1 diabetes mellitus: one year follow up. Hormones (Athens). 2015 Apr-Jun;14(2):232-40. www.ncbi.nlm.nih.gov/pubmed/25402375
39. Craig EM, Craig J, DabeleaD,Balde N, Seth A, Donaghue KC. ISPAD Clinical Practice Consensus Guidelines 2014 Compendium. Definition, epidemiology, and classification of diabetes in children and adolescents. http://onlinelibrary.wiley.com/doi/10.1111/pedi.12186/abstract
40. Li L, Jick S, Breitenstein S, Michel A. Prevalence of Diabetes and Diabetic Nephropathy in a Large U.S. Commercially Insured Pediatric Population,
2002–2013. Diabetes Care 2016 Feb; 39(2):278-284. http://care.diabetesjournals.org/content/39/2/278
41. Trotta D, Verrotti A, Salladini C, Chiarelli F.
Diabetic neuropathy in children and adolescents. Pediatr Diabetes. 2004 Mar;5(1):44-57. http://www.ncbi.nlm.nih.gov/pubmed/15043690
42. Hamid A, Wharton HM, Mills A, Gibson JM, Clarke M, Dodson PM. Diagnosis of retinopathy in children younger than 12 years of age: implications for the diabetic eye screening guidelines in the UK. Eye (Lond). 2016 Apr 15. http://www.ncbi.nlm.nih.gov/pubmed/27080488
43. Hermann G et al. Comorbidity of Type 1 Diabetes and Juvenile Idiopathic Arthritis. April 2015 Volume 166, Issue 4, Pages 930–935.e3. http://www.jpeds.com/article/S0022-3476(14)01195-0/fulltext
44. Mantravadi M. Pediatric Osteoporosis, 2016. http://emedicine.medscape.com/article/985221-overview#a3
45. Lalla E et al. Periodontal Changes in Children and Adolescents With Diabetes. Diabetes Care 2006 Feb; 29(2): 295-299. http://care.diabetesjournals.org/content/29/2/295
46. Xavier AC, Silva IN, Costa Fde O, Corrêa DS. Periodontal status in children and adolescents with type 1 diabetes mellitus. Arq Bras EndocrinolMetabol. 2009 Apr;53(3):348-54. http://www.ncbi.nlm.nih.gov/pubmed/19578597
47. American Diabetes Association. Skin Complications. www.diabetes.org/living-with-diabetes/complications/skin-complications
48. Kowalewska B, Kawko M, Zorena K, Myśliwiec M. Yeast-like fungi in the gastrointestinal tract in children and adolescents with diabetes type 1. PediatrEndocrinol Diabetes Metab. 2015;20(4):170-7. http://www.ncbi.nlm.nih.gov/pubmed/26615584
49. Kowalewska B, Zorena K, Szmigiero-Kawko M, Wąż P, Myśliwiec M. Higher diversity in fungal species discriminates children with type 1 diabetes mellitus from healthy control. Patients Prefer Adherence. 2016; 10:591-599. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4844445/
50. FDA, Food and Drug administration. Code of Federal Regulation. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=310.517
51. Onge ES, Miller SA, Motycka C, DeBerry A. A review of the treatment of type 2 diabetes in children. J PediatrPharmacolTher. 2015 Jan-Feb;20(1):4-16. http://www.ncbi.nlm.nih.gov/pubmed/25859165
52. Cherubini V, Pintaudi B, Iannilli A, Pambianchi M, Ferrito L, Nicolucci A. Long-acting Insulin Analogs Effect on gh/igf Axis of Children with Type 1 Diabetes: a Randomized, Open-label, Two-period, Cross-over Trial. ExpClinEndocrinol Diabetes. 2016 Mar 29. http://www.ncbi.nlm.nih.gov/pubmed/27023008
53. Cope JU, Samuels-Reid JH, Morrison AE. Pediatric Use of Insulin Pump Technology: A Retrospective Study of Adverse Events in Children Ages 1–12 Years J Diabetes Sci Technol.2012 Sep; 6(5): 1053–1059. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3570839/
54. Gao YQ , Gao M, Xue Y. Treatment of diabetes in children. ExpTher Med. 2016 Apr;11(4):1168-1172 http://www.ncbi.nlm.nih.gov/pubmed/27073417
55. Libman IM et al. Effect of Metformin Added to Insulin on Glycemic Control Among Overweight/ Obese Adolescents With Type 1 Diabetes: A Randomized Clinical Trial. JAMA. 2015 Dec 1;314(21):2241-50. http://www.ncbi.nlm.nih.gov/pubmed/26624824
56. Lysy J, Israeli E, Goldin E. The prevalence of chronic diarrhea among diabetic patients. Am J Gastroenterol. 1999 Aug;94(8):2165-70.
57. Pozzo AM. Pediatric Type 2 Diabetes Mellitus Medication. Medscape, May 2014. http://reference.medscape.com/article/925700-medication
58. Wen YW, Tsai YW, Huang WF, Hsiao FY, Chen PF.The potentially inappropriate prescription of new drug: thiazolidinediones for patients with type II diabetes in Taiwan. Pharmacoepidemiol Drug Saf. 2011 Jan;20(1):20-9. http://www.ncbi.nlm.nih.gov/pubmed/21182151
59. Ahuja V, Chou CH. Novel Therapeutics for Diabetes: Uptake, Usage Trends, and Comparative Effectiveness. CurrDiab Rep. 2016 Jun;16(6):47. http://www.ncbi.nlm.nih.gov/pubmed/27076180
60. Onge ES, Miller SA, Motycka C, DeBerry A. A review of the treatment of type 2 diabetes in children. J PediatrPharmacolTher. 2015 Jan-Feb;20(1):4-16. http://www.ncbi.nlm.nih.gov/pubmed/25859165
61. FDA, Food and Drug administration. Code of Federal Regulation. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=310.517
62. Yaffe S. Rational Therapeutics for Infants and Children: Workshop Summary. Similarities and Dissimilarities in Physiology, Metabolism, and Disease States and Responses to Therapy in Children and Adults. National Academies Press (US); 2000.
63. Hines RN. Ontogeny of human hepatic cytochromes P450. J BiochemMolToxicol 21:169–175, 2007. http://onlinelibrary.wiley.com/doi/10.1002/jbt.20179/abstract
64. Sahota PS, Popp JA, Hardisty JF, Gopinath G. Toxicologic pathology non clinical safety assessment. Exocrine pancreas. 2013 CRC Press, Taylor & Francis Group.
65. Ulrich AB, Standop J, Schmied BM, Schneider MB, Lawson TA, Pour PM. Species Differences in the Distribution of Drug-Metabolizing Enzymes in the Pancreas. Toxicologic Pathology, vol 30, no 2, pp 247–253, 2002. http://tpx.sagepub.com/content/30/2/247
66. WHO, World Health Organization.Micronutrients.
http://www.who.int/nutrition/topics/micronutrients/en/
67. Dr. Rath Research Institute www.drrathresearch.org
68. NIH, National Institutes of Health. Vitamin A Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/
69. Meerza D, Iqbal S, Zaheer S, Naseem I. Retinoids have therapeutic action in type 2 diabetes. Nutrition 2016 Jul-Aug;32(7-8):898-903. http://www.ncbi.nlm.nih.gov/pubmed/27134203
70. Iqbal S, Naseem I. Role of vitamin A in type 2 diabetes mellitus biology: Effects of intervention therapy in a deficient state. Nutrition. July– August, 2015 Volume 31, Issues 7-8, Pages 901–907. http://www.nutritionjrnl.com/article/S0899-9007(14)00552-8/fulltext
71. Trasino SE, Benoit DY, Gudas LJ. Vitamin A Deficiency Causes Hyperglycemia and Loss of Pancreatic β-Cell Mass. J BiolChemistry. 2015, 290(3), 1456-1473. http://www.ncbi.nlm.nih.gov/pubmed/25451926
72. NIH, National Institutes of Health. Vitamin B12 Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/
73. Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J. DiabMetab Disorders 2013; 12:7. https://jdmdonline.biomedcentral.com/articles/10.1186/2251-6581-12-17
74. Ho M et al. Vitamin B12 in obese adolescents with clinical features of insulin resistance. Nutrients. 2014 Dec 4;6(12):5611-8. https://www.ncbi.nlm.nih.gov/pubmed/25486369
75. Damião CP et al. Prevalence of vitamin B12 deficiency in type 2 diabetic patients using metformin: a cross-sectional study. Sao Paulo Med J. 2016 Jun 3. http://www.ncbi.nlm.nih.gov/pubmed/27276084
76. Akinlade KS, Agbebaku SO, Rahamon SK, Balogun WO. Vitamin B12 levels in patients with type 2 diabetes mellitus on metformin. Ann IbPostgrad Med. 2015 Dec;13(2):79-83. http://www.ncbi.nlm.nih.gov/pubmed/27162518
77. Dekelbab B, Kakkannat A. Prevalence of Vitamin B12 Deficiency in Children and Adolescents Treated with Metformin. Endocrine Society’s
96th Annual Meeting and Expo, June 21–24, 2014 – Chicago.
78. NIH, National Institutes of Health. Vitamin C Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/#h2
79. Tu H et al. Low Red Blood Cell Vitamin C Concentrations Induce Red Blood Cell Fragility: A Link to Diabetes Via Glucose, Glucose Transporters, and Dehydroascorbic Acid. EBioMedicine. 2015 Oct 3;2(11):1735-50. http://www.ncbi.nlm.nih.gov/pubmed/26870799
80. Li L, Jick S, Breitenstein S, Michel A. Prevalence of Diabetes and Diabetic Nephropathy in a Large U.S. Commercially Insured Pediatric Population,
2002–2013. Diabetes Care 2016 Feb; 39(2): 278-284. http://care.diabetesjournals.org/content/39/2/278
81. Christie-David DJ, Girgis CM, Gunton JE.
Effects of vitamin C and D in type 2 diabetes mellitus, Nutr and Dietary Suppl 2015, 15(7), 21-28. www.researchgate.net/publication/272092706_Effects_of_vitamins_C_and_D_in_type_2_diabetes_mellitus
82. Chou ST, Tseng ST. Oxidative stress markers in type 2 diabetes patients with diabetic nephropathy. ClinExpNephrol. 2016 May 27. http://www.ncbi.nlm.nih.gov/pubmed/27233502
83. ArdekaniMA ,Ardekani AS. Effect of vitamin C on blood glucose, serum lipids & serum insulin in type II diabetes patients. Indian J Med Research, vol. 126, no. 5, pp. 471–474, 2007. http://www.ncbi.nlm.nih.gov/pubmed/18160753
84. Donin AS et al. Fruit, vegetable and vitamin C intakes and plasma vitamin C: cross-sectional associations with insulin resistance and glycaemia in 9-10 year-old children. Diabet Med. 2016 Mar;33(3):307-15. http://www.ncbi.nlm.nih.gov/pubmed/26498636
85. NIH, National Institutes of Health. Vitamin D Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
86. Mosso C, Hodgson MI, Ortiz T, Reyes ML.
Bone mineral density in young Chilean patients with type 1 diabetes mellitus. J PediatrEndocrinolMetab. 2016 Jun 1;29(6):731-6. http://www.ncbi.nlm.nih.gov/pubmed/27054593
87. Al Sawah S, Compher CW, Hanlon AL, Lipman TH. 25-Hydroxyvitamin D and glycemic control: A cross-sectional study of children and adolescents with type 1 diabetes. Diabetes Res ClinPract. 2016 May;115:54-9. http://www.ncbi.nlm.nih.gov/pubmed/27242123
88. Mohammadian S, Fatahi N, Zaeri H, Ali Vakili M. Effect of Vitamin D3 supplement in glycemic control of pediatrics with type 1 diabetes mellitus and vitamin D deficiency.J Clin Diagn Res 2015, 9(3), SC05-SC07. https://www.ncbi.nlm.nih.gov/pubmed/25954674
89. He R et al. Vitamin D deficiency increases the risk of peripheral neuropathy in Chinese patients with Type 2 diabetes. Diabetes Metab Res Rev. 2016 May 7. http://www.ncbi.nlm.nih.gov/pubmed/27155442
90. Kaur H et al. Vitamin D deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. Diabetes Care. 2011 Jun;34(6):1400-2. https://www.ncbi.nlm.nih.gov/pubmed/21515836
91. NIH, National Institutes of Health. Vitamin K Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminK-HealthProfessional/#h3
92. Choi HJ et al. Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: a placebo-controlled trial. Diabetes Care. 2011 Sep;34(9):e147. https://www.ncbi.nlm.nih.gov/pubmed/21868771
93. Manna P, Kalita J. Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: A review. Nutrition. 2016 Jul-Aug;32(7-8):732-9. http://www.ncbi.nlm.nih.gov/pubmed/27133809
94. DiNicolantonio JJ, Bhutani J, O’Keefe JH. The health benefits of vitamin K. Open Heart. 2015 Oct 6;2(1):e000300. http://www.ncbi.nlm.nih.gov/pubmed/26468402
95. NIH, National Institutes of Health. Chromium Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Chromium-HealthProfessional/
96. Karagun BS et al.Chromium levels in healthy and newly diagnosed type 1 diabetic children. Pediatr Int. 2012 54(6):780-785. https://www.ncbi.nlm.nih.gov/pubmed/22783884
97. McIver DJ, Grizales AM, Brownstein JS, Goldfine AB. Risk of Type 2 Diabetes Is Lower in US Adults Taking Chromium-Containing Supplements. J Nutr. 2015 Dec;145(12):2675-82. http://www.ncbi.nlm.nih.gov/pubmed/26446484
98. NIH, National Institutes of Health. Iron Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
99. Mojs E, Stanisławska-Kubiak M, Wójciak RW, Wojciechowska J, Przewoźniak S. Reduced iron parameters and cognitive processes in children and adolescents with DM1 compared to those with standard parameters. J Investig Med. 2016 Mar;64(3):782-5. http://www.ncbi.nlm.nih.gov/pubmed/26912011
100. NIH, National Institutes of Health. Magnesium Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
101. Lin CC, Tsweng GJ, Lee CF, Chen BH, Huang YL. Magnesium, zinc, and chromium levels in children, adolescents, and young adults with type 1 diabetes. Clin. Nutr2016; 35, 880-884. https://www.ncbi.nlm.nih.gov/pubmed/26096861
102. Galli-Tsinopoulou A et al. Association between magnesium concentration and HbA1c in children and adolescents with type 1 diabetes mellitus. J Diabetes, 2014; 6(4), 369-377. https://www.ncbi.nlm.nih.gov/pubmed/24393429
103. Matthiesen G, Olofsson K, Rudnicki M. Ionized magnesium in Danish children with type 1 diabetes. Diabetes Care 2004, 27(3), 1216-1217. http://care.diabetesjournals.org/content/27/5/1216
104. Uğurlu V, Binay Ç, Şimşek E, Bal C. Cellular Trace Element Changes in Type 1 Diabetes Patients. J Clin Res PediatrEndocrinol. 2016 Jun 5;8(2):180-6. http://www.ncbi.nlm.nih.gov/pubmed/27086726
105. Huerta MG et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care 2005; 28(5), 1175-1181. http://care.diabetesjournals.org/content/28/5/1175
106. NIH, National Institutes of Health. Selenium Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
107. Özenç S et al. Selenium, zinc and copper levels and their relation with HbA1c status in children with type 1 diabetes mellitus. In t J Diabetes DevCtrs, 2015, 35(4) 514-518. http://link.springer.com/article/10.1007/s13410-015-0327-y
108. NIH, National Institutes of Health. Zinc Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/
109. Wu Y et al. Zinc stimulates glucose consumption by modulating the insulin signaling pathway in L6 myotubes: essential roles of Akt-GLUT4, GSK3β and mTOR-S6K1. J NutrBiochem. 2016 May 31;34:126-135. http://www.ncbi.nlm.nih.gov/pubmed/27295130
110. Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi GR, Katulanda P. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. DiabetolMetab Syndrome 2012; 4:13. https://www.ncbi.nlm.nih.gov/pubmed/22515411
111. Bideci A, Amurdan MO, Cinaz P, Dursun H, Demirel F. Serum zinc, insulin-like growth factor-I and insulin-like growth factor binding protein-3 levels in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18(10):1007–11. https://www.ncbi.nlm.nih.gov/pubmed/16355814
112. Angelova M, Schentov B, Nedkova V, Nikoloff G, Alexiev AI, Petrova Ch. Serum zinc in children with enterocolitis, chronic diarrhea with malabsorption syndrome and type 1 diabetes. Trakia Journal of Sciences. 2006;4:11–7
113. Tuvemo T, Ewald U, Kobbah M, Proos LA. Serum magnesium and protein concentrations during the first five years of insulin-dependent diabetes in children. Acta Paediatr. 1997;418(Suppl):7–10. https://www.ncbi.nlm.nih.gov/pubmed/9055931
114. Rohn RD, Pleban P, Jenkins LL. Magnesium, zinc and copper in plasma and blood cellular components in children with IDDM. Clin Chim Acta. 1993;215(1):21–8. https://www.ncbi.nlm.nih.gov/pubmed/8513565
115. Estakhri M et al.Serum Zinc Levels in Children and Adolescents with Type-1 Diabetes Mellitus. Iran J Public Health. 2011; 40(4): 83–88. https://www.ncbi.nlm.nih.gov/pubmed/23113106
116. Nakamura T, Higashi A, Nishiyama S, Fujimoto S Matsuda. Kinetics of Zinc Status in Children with IDDM. Diabetes Care 1991; 14(7):
553-557. https://www.ncbi.nlm.nih.gov/pubmed/1914794
117. Heneman K, Zidenberg-Cherr S. Some facts about phytochemicals. Center for Health and Nutrition Research University of California. October 2008. http://nutrition.ucdavis.edu/content/infosheets/fact-pro-phytochemical.pdf
118. Jacques PF, Cassidy A, Rogers G, Peterson JJ, Meigs JB, Dwyer JT. Higher Dietary Flavonol Intake Is Associated with Lower Incidence of Type 2 Diabetes1,2. J Nutr. 2013 Sep; 143(9): 1474–1480. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3743276/
119. Jiao H, Hu G, Gu D, Ni X. Having a promising efficacy on type II diabetes, it’s definitely a green tea time. Curr Med Chem. 2015;22(1):70-9. https://www.ncbi.nlm.nih.gov/pubmed/25139278
120. Szkudelski T, Szkudelska K.Anti-diabetic effects of resveratrol. Ann N Y Acad Sci. 2011 Jan;1215:34-9. https://www.ncbi.nlm.nih.gov/pubmed/21261639
121. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp BiochemPhysiol C ToxicolPharmacol. 2003 Jul;135C(3):357-64. https://www.ncbi.nlm.nih.gov/pubmed/12927910
122. Nabavi SF et al. Curcumin: a natural product for diabetes and its complications. CurrTop Med Chem. 2015;15(23):2445-55. https://www.ncbi.nlm.nih.gov/pubmed/26088351
123. Crawford P et al. Assessment of the effect of lifestyle intervention plus water-soluble cinnamon extract on lowering blood glucose in pre-diabetics, a randomized, double-blind, multicenter, placebo controlled trial: study protocol for a randomized controlled trial. Trials. 2016 Jan 5;17:9. http://www.ncbi.nlm.nih.gov/pubmed/26732017
124. Sahib AS. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: A randomized, placebo-controlled clinical trial. J IntercultEthnopharmacol. 2016 Feb 21;5(2):108-13. http://www.ncbi.nlm.nih.gov/pubmed/27104030
125. Im K, Issac A, NM J, Ninan E, Maliakel B, Kuttan R.Effects of the polyphenol content on the anti-diabetic activity of Cinnamomum zeylanicum extracts. Food Funct. 2014 Sep;5(9):2208-20. http://www.ncbi.nlm.nih.gov/pubmed/25051315
126. Jiménez-Osorio AS, Monroy A, Alavez S. Curcumin and insulin resistance-Molecular targets and clinical evidences. Biofactors. 2016 Jun 21. http://www.ncbi.nlm.nih.gov/pubmed/27325504
127. Weisberg S, Leibel R, Tortoriello DV. Proteasome inhibitors, including curcumin, improve pancreatic β-cell function and insulin sensitivity in diabetic mice. Nutr Diabetes. 2016 Apr 25;6:e205. doi: 10.1038/nutd.2016.13. http://www.ncbi.nlm.nih.gov/pubmed/27110686
128. Gunnink LK et al. Curcumin directly inhibits the transport activity of GLUT1. Biochimie. 2016 Jun;125:179-85. http://www.ncbi.nlm.nih.gov/pubmed/27039889
129. Anupunpisit V, Petpiboolthai H, Khimmaktong W. Microvasculature Improvement of Heart in Diabetic Rat with Curcumin Supplementation. J Med Assoc Thai. 2015 Nov;98Suppl 10:S74-83. http://www.ncbi.nlm.nih.gov/pubmed/27276836
130. Kim BH et al. Protective Effects of Curcumin on Renal Oxidative Stress and Lipid Metabolism in a Rat Model of Type 2 Diabetic Nephropathy. Yonsei Med J. 2016 May;57(3):664-73. http://www.ncbi.nlm.nih.gov/pubmed/26996567
131. Elburki MS et al. A novel chemically modified curcumin reduces inflammation-mediated connective tissue breakdown in a rat model of diabetes: periodontal and systemic effects. J Periodontal Res. 2016 Apr 1. http://www.ncbi.nlm.nih.gov/pubmed/27038334
132. Keske MA et al. Vascular and metabolic actions of the green tea polyphenol epigallocatechingallate. Curr Med Chem. 2015;22(1):59-69. http://www.ncbi.nlm.nih.gov/pubmed/25312214
133. Borges CM, Papadimitriou A, Duarte DA, Lopes de Faria JM, Lopes de Faria JB. The use of green tea polyphenols for treating residual
albuminuria in diabetic nephropathy: A double- blind randomised clinical trial. Sci Rep. 2016 Jun 20;6:28282. http://www.ncbi.nlm.nih.gov/pubmed/27320846
134. Raposo D, Morgado C, Pereira-Terra P, Tavares I.
Nociceptive spinal cord neurons of laminae I-III exhibit oxidative stress damage during diabetic neuropathy which is prevented by early antioxidant treatment with epigallocatechin- gallate (EGCG). Brain Res Bull. 2015 Jan;110:68-75. http://www.ncbi.nlm.nih.gov/pubmed/25522867
135. Eid HM, Nachar A, Thong F, Sweeney G, Haddad
PS.The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn Mag. 2015 Jan-Mar;11(41):74-81. http://www.ncbi.nlm.nih.gov/pubmed/25709214
136. Kitti M et al. Quercetin Stimulates Insulin Secretion and Reduces the Viability of Rat INS-1 Beta-Cells. Cell PhysiolBiochem. 2016 Jun 24;39(1):278-293. http://www.ncbi.nlm.nih.gov/pubmed/27336168
137. Yan SX, Li X, Sun CD, Chen KS. Hypoglycemic and hypolipidemic effects of quercetin and its glycosides ZhongguoZhong Yao ZaZhi. 2015 Dec;40(23):4560-7. http://www.ncbi.nlm.nih.gov/pubmed/27141664
138. Wong RH, Nealon RS, Scholey A, Howe PR. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. NutrMetabCardiovasc Dis. 2016 May;26(5):393-9. http://www.ncbi.nlm.nih.gov/pubmed/27105868
139. Pektaş MB et al. Resveratrol Ameliorates the
Components of Hepatic Inflammation and Apoptosis in a Rat Model of Streptozotocin-Induced Diabetes. Drug Dev Res. 2016 Feb;77(1):12-9. http://www.ncbi.nlm.nih.gov/pubmed/26748675
140. Zeng K et al. Resveratrol Prevents Retinal Dysfunction by Regulating Glutamate Transporters, Glutamine Synthetase Expression and Activity in Diabetic Retina. Neurochem Res. 2016 May;41(5):1050-64. http://www.ncbi.nlm.nih.gov/pubmed/26677078
141. NIH, National Institutes of Health. Coenzyme Q10 in depth. https://nccih.nih.gov/health/supplements/coq10
142. Tsai HY et al. Coenzyme Q10 Attenuates High
Glucose-Induced Endothelial Progenitor Cell Dysfunction through AMP-Activated Protein Kinase Pathways. Diabetes Res. 2016;2016:6384759. http://www.ncbi.nlm.nih.gov/pubmed/26682233
143. Menke T, Niklowitz P, Wiesel T, Andler W. Antioxidant level and redox status of coenzyme Q10 in the plasma and blood cells of children with diabetes mellitus type 1.Pediatr Diabetes. 2008; 9(6):540-5. https://www.ncbi.nlm.nih.gov/pubmed/18694454
144. USDA. U.S. Department of Agriculture. Dietary, functional and total fiber. 339-441. https://fnic.nal.usda.gov/sites/fnic.nal.usda.gov/files/uploads/339-421.pdf
145. Velázquez-López L et al. Fiber in Diet Is Associated with Improvement of Glycated Hemoglobin and Lipid Profile in Mexican Patients with Type 2 Diabetes.J Diabetes Res.2016;2016:2980406. http://www.ncbi.nlm.nih.gov/pubmed/27144178
146. Nansel TR, Lipsky LM, Liu A. Greater diet quality is associated with more optimal glycemic control in a longitudinal study of youth with type 1 diabetes. Am J ClinNutr. 2016 Jul;104(1):81-7. http://www.ncbi.nlm.nih.gov/pubmed/27194309
147. Moreno LA et al. Psyllium fibre and the metabolic control of obese children and adolescents. J PhysiolBiochem. 2003;59:235–242. https://www.ncbi.nlm.nih.gov/pubmed/15000455
148. Ventura EE et al. Dietary intake and the metabolic syndrome in overweight Latino children. J Am Diet Assoc 2008, 108, 1355-1359. https://www.ncbi.nlm.nih.gov/pubmed/18656576
149. McKeown NM, Meigis JB, Liu S, Satzman E, Wilson PW, Jacques PF. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 2004; 27, 538-546. https://www.ncbi.nlm.nih.gov/pubmed/14747241
150. Cha JC, Ivanov V, Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M. Nutritional improvement of metabolic syndrome parameters in immature fructose-fed wild-type mice. Mol Med Rep. 2011 Nov- Dec;4(6):1053-9. http://www.ncbi.nlm.nih.gov/pubmed/21874237