Vitamin C in Health: Scientific focus on its anti-cancer efficacy
Vitamin C, also known as L-ascorbic acid, is an undisputable essential vitamin for human health with antioxidant and anti-cancer properties among others. It is a cofactor for a number of metabolic enzymes and has enormous health benefits. Extensive epidemiological, in vitro, in vivo and clinical studies consistently and strongly suggest the benefits of Vitamin C use in cancer treatment. Epidemiological studies have shown that people consuming a diet rich in Vitamin C are less likely to develop cancer. In vitro and in vivo studies have shown that vitamin C kills cancer cells while simultaneously supporting normal cells and tissues. Clinical data indicates that intravenous administration of vitamin C is much more effective in achieving higher plasma levels, compared to oral administration, making the former the preferred means of administering Vitamin C to achieve sustainable therapeutic effect in cancer treatment. There are a wide variety of mechanisms by which vitamin C kills cancer cells and prevents their spread which include its roles as an anti-oxidant, an inhibitor of metalloproteinases and a supporter of collagen formation and tissue architecture. The therapeutic effect of Vitamin C is accompanied by the lack of cytotoxicity induced by conventional chemotherapy making it the most desirable anti-cancer therapy and one of the safest substances available to physicians. It has been observed through several studies that the anti-carcinogenic characteristics of Vitamin C are further enhanced in combination with other micronutrients such as lysine, arginine, proline and green tea extract. The synergistic effect of these nutrient mixtures can thus be considered in preventive and therapeutic aspects of cancer.
Keywords
Vitamin C, ascorbic acid, cancer, epidemiology, in vitro, in vivo, clinical studies, nutrient mixture.
Correspondence to Dr. Aleksandra Niedzwiecki, Dr. Rath Research Institute, 1260 Memorex Drive, Santa Clara, CA 95050, USA. Email: author@jcmnh.org
Cancer is the second leading cause of death after heart disease in the Western World and it is estimated that 2.6 million new cancer cases will be diagnosed by 2050. Chemicals, radiation and viruses (1, 2, 3) have been recognized as cancer causing agents in human and many animal species. A wide variety of carcinogenic agents circulate in the environment contaminating air, water and food sources. Although there is great diversity in the nature of these agents, the resulting cellular response to them is the transformation of normal cells into cancer cells (Figure 1). This process of transformation primarily involves three distinct phases: initiation, promotion and progression. Cancer initiation often requires sustained DNA damage induced by free radicals in the cellular environment which either cannot be repaired or is missed by the DNA repair enzymes. The mutations in the DNA accumulate over time thereby increasing the risk of cancer with each multiplication. It is therefore no surprise that rapidly dividing cells are more susceptible to carcinogens than slowly dividing cells. Finally the stage of progression is reached when the uncontrolled multiplication of these cells forms a cell mass interfering with organ function and the cells also acquire the ability to move into other tissues. Based on the associated degree of spread cancer development shows the following stages: 1) hyperplasia (increased number of cells and division), 2) invasion to the adjacent tissues and 3) metastasis (cellular migration to distal organs) (4).
Currently surgery, chemotherapy and radiation are considered standard cancer therapies (3). Although these therapeutic approaches can initially inhibit the tumor mass, they are ineffective in providing a cure. Moreover, these treatments indiscriminately attack all cells including healthy cells causing extensive cellular damage and cytotoxicity. This gives rise to new cancers and promotes development of drug resistance in cancer cells. Furthermore, these therapies are ineffective in curtailing cancers that have already metastasized.
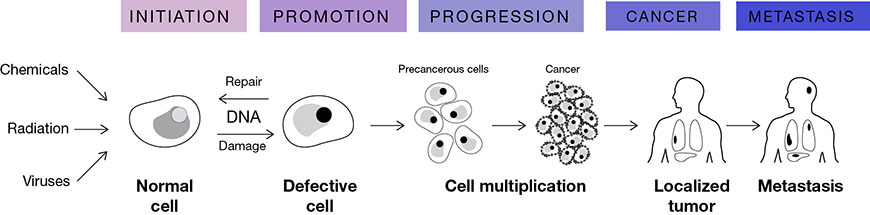
Figure 1. Cancer causing agents and the stages of transforming normal cell to cancerous cells
There is an urgent need to develop effective non-toxic anti-cancer approaches that include prevention of metastasis.
In recent decades it has been shown that vitamins, in particular vitamin C used at higher doses can be effective as therapeutic agents that target several mechanisms associated with different pathologies. Among different health applications of vitamin C, its role in relation to cancer has been most thoroughly investigated.
Vitamin C
Discovery of vitamin C. The importance of vitamin C for human health can be traced back to several centuries ago. Its deficiency causes a disease called scurvy which is characterized by bleeding gums, dry skin, fragile blood vessels, connective tissue dissolution, impaired wound healing and finally death (5, 6). Interestingly scurvy is absent in most species of the animal world and can develop only in humans, guinea pigs and primates, species that have lost the ability to internally produce vitamin C.
The earliest documented case of scurvy was described by Hippocrates around the year 400 BC. This disease became rampant during the 16th century among sea voyageurs and sailors who were deprived of fresh fruits and vegetables in their diet during long voyages. The first clinical proof that citrus fruit juice can cure scurvy was provided by Dr. James Lind who published his findings in the ‘Treatise of the Scurvy’ in 1753 (7).
However, it was not until the 1900s, after the establishment of the first guinea pig scurvy model (8), that this antiscorbutic factor was identified as “water-soluble vitamin C” or “ascorbic acid”. This compound was isolated and its structure identified (Figure 2) by Hungarian scientist Albert Szent-Gyorgyi. In 1933, Hoffmann La Roche became the first pharmaceutical company responsible for the mass production of vitamin C. The role of vitamin C as an anti-cancer agent was first suggested in 1952 when McCormick proposed its role as a chemotherapeutic agent (9)
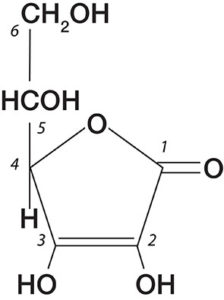
Figure 2. Structure of Vitamin C (Ascorbic Acid)
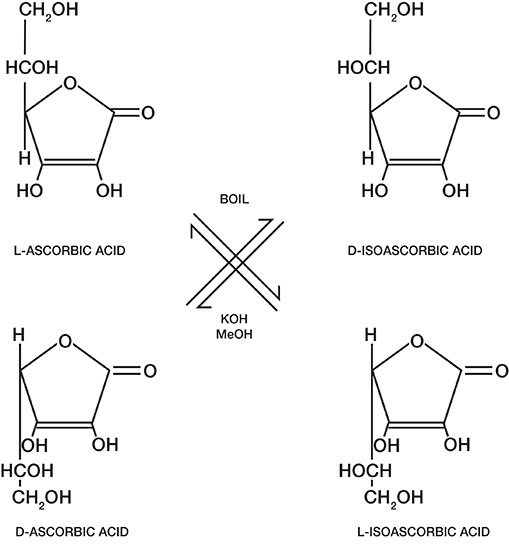
Figure 3. Stereoisomers of Vitamin C and their interconversion
Chemical structure. Natural vitamin C is also known as L-ascorbic acid (Figure 3). The molecule has two asymmetric carbon atoms that result in four stereoisomers: L- Ascorbic acid, D-Ascorbic Acid, D-iso Ascorbic Acid and L-iso Ascorbic Acid. Of the four isomers, only L-Ascorbic Acid or vitamin C is biologically active and the other three forms are inactive.
Vitamin C is a colorless, odorless, crystalline solid compound with a sharp acidic taste. Its chemical composition is C6H8O6, with a molecular weight of 176 and a melting point of 190-1920C. It is soluble in water but insoluble in organic solvents. Vitamin C is easily oxidized by several agents including halogens and hydrogen peroxide (10, 11).
The Vitamin C molecule contains several reactive hydroxyl groups, especially at the 2- and 6- positions allowing a variety of derivatives to be easily synthesized. 2- sulfate and 2- phosphate compounds of vitamin C are much more stable than vitamin C itself, hence they are used in formulations of feed for shrimp, fish, guinea pigs and rhesus monkeys. L-ascorbyl 6-palmitate, a synthetic lipophilic vitamin C derivative is another effective and bio-available compound used in foods and pharmaceuticals (10, 11). It has been seen that both hydrophilic and hydrophobic derivatives are cytotoxic to a number of malignant cells.
Sources and distribution. Vitamin C is a ubiquitous compound necessary for sustaining life and it is considered one of the most versatile and vital dietary compounds (10). It is widely available in fresh fruits and vegetables. Fruits such as oranges, lemons, grapes, strawberries, papaya, kiwi, cantaloupe, grapefruit, mango and honeydews are good sources of Vitamin C (10, 11, 12). Vegetables rich in vitamin C include broccoli, Brussels sprouts, red and green peppers, tomatoes, cabbage, potatoes, sweet potatoes, cauliflower, snow peas and kale. Cooking generally destroys vitamin C as does storage since the compound is unstable and extremely sensitive to heat. It is also easily oxidized and can be destroyed by exposure to oxygen, alkali, iron and copper (13, 14, 15). Thus in order to preserve vitamin C the food should not be exposed to air, light or water for prolonged periods of time (10, 11).
Vitamin C is concentrated in many organs in humans and animals. Its highest levels are found in the adrenal and pituitary glands. Other organs rich in vitamin C include the liver, spleen, pancreas, kidney, brain, thymus, and the lens of the eye. By virtue of its mass, the liver stores the greatest amount of vitamin C followed by the thymus, brain and pancreas (10, 11, 12).
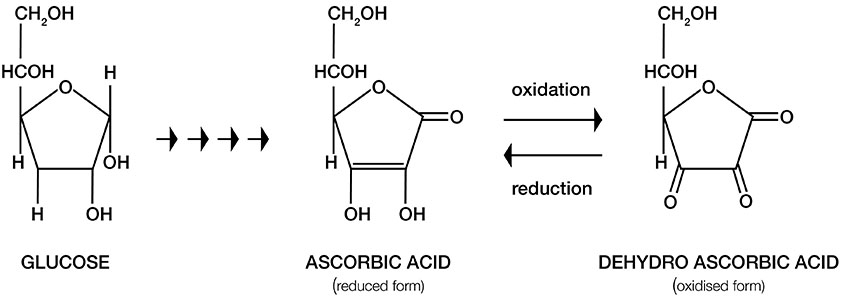
Figure 4. Metabolic pathway of vitamin C synthesis from glucose. Ascorbic acid (AA) and its oxidized form Dehydroascorbic acid (DHA) have similar structures.
Bio-synthesis. Most animals and plants synthesize vitamin C from glucose (16) (Figure 4). In primitive fish, amphibians and reptiles, vitamin C is synthesized in the kidney, whereas the liver is the main site of vitamin C production in most mammals. Humans, monkeys, guinea pigs, fruit eating bats and several other primates have lost the ability to synthesize vitamin C endogenously as a result of genetic mutations that impair the ability to produce L-gulonolactone oxidase( GULO) – the enzyme that catalyzes a critical step in conversion of glucose to vitamin C.
Cellular uptake. Vitamin C is easily oxidized and converted to dehydroascorbic acid (DHA) on exposure to air. In vitro studies have found that oxidized vitamin C (DHA) enters the cell via nonspecific, low-affinity, high-capacity glucose transporters (GLUTs – hexose transporters) and is reduced intracellularly to L- ascorbic acid. L-ascorbic acid is also directly taken up by the cells through specific, high-affinity, low-capacity sodium-ascorbate transporters (SVCTs – sodium-ascorbate co-transporter). Both transport mechanisms exist in the majority of cells in the body (Figure 5) (17, 18, 19, 20).
Function and physiological relevance. Vitamin C is needed for hundreds of biochemical reactions occurring in the body and for the growth and repair of tissues.
One of the main roles Vitamin C plays is that of an anti-oxidant. One of the agents that cause extensive damage to cells and cellular components are free radicals which are highly reactive molecules containing a single electron. Free radicals are generated both during normal cellular metabolism and in pathological conditions. Free radical induced damage to cellular proteins, fats and carbohydrate molecules and their buildup over time contributes to the aging process as well as the development of many pathologies. The most known radical compounds include superoxide, hydroxyl and singlet oxygen, ozone, peroxynitrile, nitrogen dioxide and hypochlorus acid (21, 22).
Protection from free radicals is provided by compounds with antioxidant properties that can scavenge the radicals. Vitamin C is considered the most effective, least toxic water soluble antioxidant. It is the predominant antioxidant in blood, tissues and intracellular fluids (23, 24). Due to its antioxidant function Vitamin C helps protect several macromolecules such as DNA, lipids, proteins and enzymes from damage by free radicals. Consequently, it offers protection from degenerative diseases such as cancer, aging, heart disease and cataract formation.
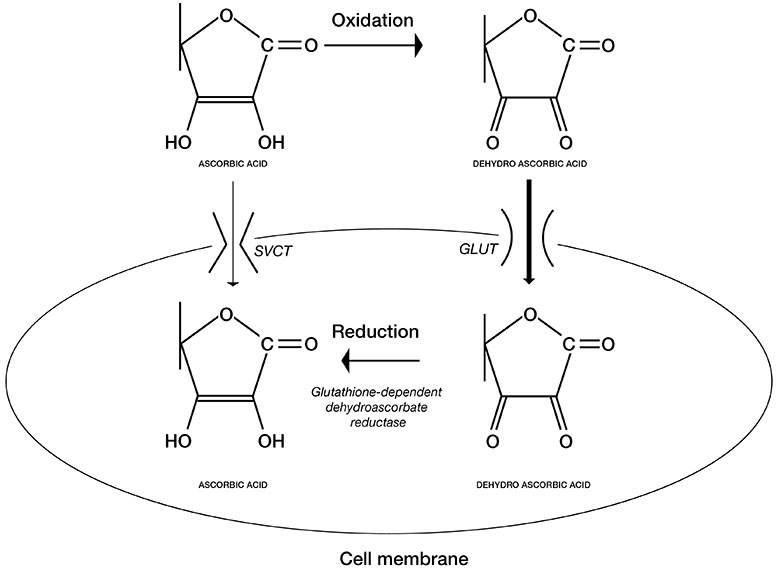
Figure 5. Cellular uptake of Vitamin C and its oxidized form, dehydroascorbic acid (DHA) through different transporters.
In vivo studies have shown that L-Ascorbic Acid is utilized in cell metabolism as an electron donor capable of donating one or two electrons. In the first step of oxidation, an ascorbyl radical is generated followed by the formation of dehydroascorbic acid in the second step. The ascorbyl radical does not accumulate in vivo, because two ascorbyl radicals dismutate into one ascorbic acid molecule and one dehyroascorbic acid molecule. (Figure 5).
Besides its antioxidant function vitamin C plays an indispensable role in maintaining tissue structure and function by regulating the biosynthesis and structure of collagen (25), which is one of the main structural components of blood vessels, skin, tendons, ligaments, bone, cartilage, heart valves and lens of the eye. Optimum collagen formation is also essential in healing wounds and assembly of the extracellular matrix. Vitamin C transcriptionally regulates synthesis of collagen and is also an essential co-factor of Lysyl- and Prolyl- hydroxylases, the enzymes catalyzing formation of hydrogen bridges which link various collagen fibrils. Besides collagen, Vitamin C also plays a role in the synthesis of glycosaminoglycans which are essential components of cartilage, bone and connective tissue. Thus wound healing and maintenance of tissue and organ structure and function are processes dependent on the availability of Vitamin C (10, 11, 12, 21, 22).
Vitamin C also participates in the biosynthesis of hormones, carnitine, and neurotransmitters. It also helps the body to absorb iron from non-heme sources and helps keep metals such copper and iron in a reduced state which is essential for many biological reactions in the human body. It also recycles other antioxidants such as vitamin E and glutathione. Vitamin C is an effective antimicrobial agent inhibiting viral and bacterial infections in the body. Other than that health conditions such as high blood pressure, diabetes, asthma & other allergic conditions, gout, infertility, schizophrenia and depression are affected by vitamin C (10, 11, 21).
Vitamin C and Cancer
Interest in the chemo-preventive functions of vitamin C and other antioxidants (10, 11, 26) has grown considerably in recent years since conventional therapies such as chemotherapy and radiation therapy have been unable to provide a cure for cancer. Chemotherapeutic compounds and radiation indiscriminately attack cancer cells and healthy cells causing severe cellular damage including damage to the body’s connective tissues and immune system. This cellular damage leads to new cancers, infections, severe anemia, impaired immunity and bleeding. Furthermore, these therapies are ineffective in treating cancers diagnosed at a late stage when metastasis has already occurred.
Interest in Vitamin C has arisen since several epidemiological, cell culture, animal and human studies consistently and strongly suggest that it has a protective effect against cancer.
Epidemiological studies. An extensive body of evidence accumulated over the years has shown that people consuming diets rich in fruits and vegetables are less likely to develop cancer than people who have lower intake of these foods (27, 28, 29). The US Department of Agriculture and the NCI recommends the consumption of at least 5 servings of fruits and vegetables per day to prevent cancer (30).
While many phytochemicals and micronutrients in fruits and vegetables may have anticancer properties, vitamin C is likely to be one of the most effective anti-carcinogenic agents. Its anti-carcinogenic properties can be attributed to its antioxidant properties such as preventing free radical damage to DNA, decreasing the formation of carcinogenic nitrosamines and mutagens, enhancing the immune system to fight cancer cells, accelerating the action of detoxifying liver enzymes and blocking the toxic effects of carcinogens (e.g. polycyclic hydrocarbons, organochlorine pesticides, and heavy metals). Epidemiological findings have consistently established a correlation between a high intake of vitamin C (or food rich in vitamin C) and a reduced risk of stomach cancer. This may be due to the action of vitamin C blocking the formation of nitrosamine and other carcinogens in the stomach.
Several epidemiological studies have examined the role of vitamin C or vitamin C rich foods in cancer prevention, the vast majority of which demonstrate a statistically significant protective effect. A number of studies have investigated and demonstrated the preventive role of vitamin C in cancers of the mouth, esophagus, oral cavity, larynx, stomach and pancreas (27, 28). There is also substantial evidence of its protective effect against cancers of the cervix, rectum and breast. Several recent lung cancer studies suggest significant protective effects of vitamin C or of food rich in vitamin C.
Cell culture studies. In recent years there has been growing interest in the therapeutic application of vitamin C and its derivatives (26, 31, 32, 33, 34, 35, 36). Much of the evidence for the anti-cancer efficacy of vitamin C comes from cell culture studies that investigate the therapeutic potential of vitamin C and its derivatives directly on cancer cells (26, 31, 36).
There is increasing evidence that vitamin C is selectively toxic to some types of cancer cells, thereby functioning as a pro-oxidant. It has been reported that it is cytotoxic to some human cancers such as neuroblastoma, osteosarcoma and retinoblastoma. At concentrations ranging from 10 nM to 1 mM vitamin C induces apoptosis in neuroblastoma and melanoma cells (11). It also acts as a modulator of growth in mouse myeloma cells as seen in an in vitro colony assay and human bone marrow cells. Vitamin C is also highly toxic to Ehrlich ascites carcinoma cells and 3T3 cells in culture (26).
While at low concentrations, vitamin C was found to be cytotoxic to mouse lymphocytic leukemia cells, mouse cells from neoplasms, and acute lymphoblastic leukemia human cell lines (36, 37, 38), at much higher concentrations it is also toxic to malignant cell lines. Japanese scientists have shown the anti-cancer effect of benzylidene ascorbate against human tumors of the ovary, stomach, pancreas, uterus, bile ducts and lungs (39). Benzylidene ascorbate has also been shown to induce apoptosis, or cell death, in human myelogenous leukemic cell lines, rat hepatocellular carcinoma cells, and in an HIV-replicating human lymphoma cell line. Its cytotoxic effect has been related to its pro-oxidant activity and activation of transcriptional factor NF-kappa B (26, 39).
A number of vitamin C isomers and derivatives were synthesized and tested for their anti-cancer effects in vitro and it was demonstrated that derivatives with substitutions at -2 or -6 and at both positions have a marked cytotoxic effect. For instance, Ascorbate 6-palmitate and 6-stearate, were found to be more potent inhibitors of murine leukemia cell proliferation compared to ascorbate 2-phosphate, and ascorbate 6-phosphate or ascorbate 6-sulfate respectively (40). Ascorbate 6- palmitate and ascorbate 6-stearate have also been shown to inhibit the proliferation of mouse gliomas, human gliomas, U-373 & T98G cells and renal carcinomas. All of these were attributed to inhibition of cell proliferation, cell cycle arrest and apoptosis (11, 41).
Park et al. (37, 38, 39) and Chen et al. (42, 43) studied the effect of vitamin C on a number of cancer cell lines as summarized in Table 1. Their results demonstrate that vitamin C at differing concentrations may utilize varying mechanisms to target cancer cells. At concentrations of 0.25 mM – 1.0 mM Vitamin C induces a dose and time dependent cancer cell inhibition due to oxidative stress. Treatment of cells with high doses of vitamin C resulted in increased intracellular GSH (glutathione) and GSH-S transferase activity that was accompanied by an uptake of cysteine thereby suggesting that high concentrations of vitamin C can modulate intracellular sulfur containing compounds such as glutathione and cysteine.
In their pharmacokinetic studies in humans, Chen et al. (42, 43) investigated whether pharmacological concentrations of ascorbate can kill cancer cells selectively. The study tested toxicity of vitamin C towards several cancer cell lines and normal cell. The results indicated that the intravenous administration of ascorbate (5 mM – 10 mM for 1-2hrs) selectively induced death in 75% of the 48 cancer cell lines tested, but demonstrated no toxic effects on human peripheral white blood cells, fibroblasts and epithelial cells.
Most recently, the study by Yun et al. shed more light on anti-cancer mechanism of vitamin C. The researchers used cancer cells with BRAF and KRAS mutations which make unusually high levels of a protein that transports glucose across the cell membrane (49). Glucose and vitamin C share the same transporter – GLUT1. The results showed that large doses of vitamin C kill these mutated cells by raising the free radical levels, which in turn inactivate an enzyme needed to metabolize glucose, thereby depriving these cells of energy.
Table 1. Effect of vitamin C on cell survival.
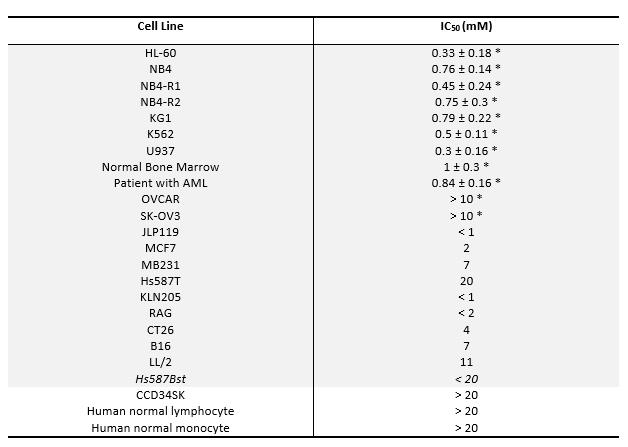
IC50: the concentration of the drugs which causes inhibition of cellular proliferation in 50 % of the cells. The IC50 values are means ± standard deviations from triplicate experiments. HL-60, human myeloid leukemia; NB4, NB4-R1, NB4-R2, human acute promyelocytic leukemia (APL); KG1, human myeloblast; K562, human chronic myelogenous leukemia; U937, human histiocytic lymphoma; OVCAR, SK-OV3, ovarian cancer; JLP119, human lymphoma; MCF7, MB231, Hs587t, human breast cancer; KLN205, mouse lung cancer; RAG, mouse kidney cancer; CT26, mouse colon cancer; B16, mouse melanoma; LL/2, mouse lung cancer; Hs587Bst, human normal breast cells; CCD34SK, human normal fibroblast cells. *IC50 value was determined using H3 incorporation proliferation assay for 24h.
Animal studies. Most of the beneficial effects of vitamin C in cancer observed in in vitro studies have been supported by in vivo testing as well (26, 44). Studies by Linus Pauling and his colleagues have shown that high dietary intake of vitamin C decreases the incidence and delays the onset of malignant skin tumors in mice initiated by exposure to ultraviolet light (45). It also delays the onset of spontaneous mammary tumors. A mixture of vitamin C and cupric sulfate administered orally significantly inhibited human mammary tumor growth in mice (43, 45, 46). Vitamin C also decreased the incidence of kidney tumors generated by estradiol or diethylstilbesterol treatment in hamsters (47) due to a decrease in the formation of genotoxic metabolites. Other investigators have also found that vitamin C and its lipophilic derivatives such as ascorbyl palmitate, are effective in preventing skin cancer. Development of colon, kidney and bladder cancer in animals can also be controlled by vitamin C intake. Significant inhibition has also been observed with vitamin C treatment in the development of lung cancer in mice exposed to fiberglass dust (44).
Our study conducted in mice lacking the ability to synthesize endogenous vitamin C (GULO-/-) has shown that animals supplemented with vitamin C develop lesser number of tumors, which in turn are encapsulated by a layer of connective tissue rendering them less invasive. Tumor metastasis in these mice was reduced by 71% (48).
In a study by Levine et al, following a regimen of daily pharmacological ascorbate doses (4g/day) significantly decreased the growth rate of ovarian, pancreatic and glioblastoma tumors in mice (46). High concentrations of vitamin C also inhibited tumor growth in Balb/C mice implanted with sarcoma 180 cancer cell lines (39). The survival rate in the group that received high doses of vitamin C increased by 20% compared to the control group.
A study conducted by scientists at John Hopkins University (49) showed that colon cancer cells carrying mutations in KRAS or BRAF genes can specifically accumulate DHA. The increased intracellular DHA can in turn induce oxidative stress by depleting intracellular glutathione thereby increasing the vulnerability of cancer cells to free radical damage and death. As expected, Apc/KrasG12D mutant mice exposed to high doses of Vitamin C developed fewer and smaller tumors.
Besides directly lowering the incidence and metastasis of several types of cancers vitamin C also demonstrates a supportive role for the normal tissues. Its beneficial effect on normal cells also helps in decreasing the cytotoxic side effects of radiation and chemotherapeutic agents when Vitamin C is used as an adjuvant.
Clinical studies. Cancer patients typically have significantly reduced levels of vitamin C in serum, compared to healthy people. Large doses of vitamin C can correct these serum levels and improve immunity and other physiological functions (22, 27, 29).
Clinical studies conducted by Cameron and his associates in Scotland (50); and later by Linus Pauling (51, 52) showed that on an average, terminal cancer patients who were prescribed 10g/day of vitamin C survived significantly longer than similar cancer patients who did not get a vitamin C supplement. Tumor regression was also reported in some patients with cancer of the lung, pancreas, small intestine, colon, breast or kidney (44). Cameron and Pauling observed no response or minimal response to vitamin C in about 45% of patients but the majority of patients experienced therapeutic responses of tumor retardation, cytostasis, or regression. Patients also reported a greater sense of well-being, improved appetite, increased mental alertness & physical strength and a decreased requirement for pain-killing drugs. These studies indicated that vitamin C therapy is more effective when started at an earlier than later stage. The results were also confirmed by Japanese researchers who found similar survival times (53, 54).
Subsequently, Canadian scientist Dr. Abram Hoffer (55) provided more evidence that vitamin C enables cancer patients to live longer with a better quality of life. However, Cameron and Pauling’s work on vitamin C suffered a setback from subsequent studies, including a randomized, double-blind, placebo-controlled clinical trial by Dr. Charles Moertel of the Mayo Clinic (56). Interestingly the two studies differed in the modes of administration of Vitamin C, with Pauling recommending both intravenous and oral administration, and Moertel only administering orally thereby limiting anti-cancer efficacy of vitamin C.
However, subsequent pharmacokinetic analysis with oral and combined oral/intravenous administration of Vitamin C by Levine (42, 43, 46) and Riordan (59, 60, 61, 62) suitably explained the conflicting findings. Oral intake of vitamin C, even at a very large dose, can raise plasma vitamin C concentrations to a maximum of only 200 μm/L; whereas an intravenous administration can raise plasma concentrations to as high as 26,000 μm/L. Concentrations of this magnitude can selectively kill tumor cells in vitro without any adverse effect to normal human cells. When 0.25-0.5 g/kg vitamin C was injected into rats intravenously or intraperitoneally, it produced an ascorbate concentration of 60-100 fold higher in blood and tissue than with the same oral dose. These findings were soon followed up by many laboratories all over the world.
A number of studies thereafter have shown that high doses of parenteral ascorbate inhibits the growth of xenografts of a number of cancer cell lines in mice such as glioblastoma, pancreatic cancer, breast cancer and neuroblastoma. In vitro and in vivo studies from several groups have also demonstrated the synergistic effect of pharmacologic ascorbate doses when combined with common chemotherapeutic drugs such gemcitabine, paclitaxel and carboplatin (44). Amelioration of adverse side effects of chemotherapy and improvement in quality of life has been demonstrated in breast cancer patients too.
In several studies, Riordan et al. clearly indicated benefits of vitamin C intake when used as an adjuvant with chemotherapy. Dr. Kedar Prasad showed that high doses of vitamin C as well as other antioxidants can protect healthy cells during chemotherapy treatment (26). He further states that cancer cells unlike healthy cells cannot regulate vitamin C uptake which results in their death. This study was further supported by the findings from UCLA and MD Anderson Cancer Center advocating that antioxidants should be taken during cancer therapy.
Since administration of vitamin C enhances iron absorption, iron overload must be ruled out during treatment. Intravenous injection of vitamin C can also create a high load of sodium which can result in fluid overload in patients with congestive heart failure, renal insufficiency or renal failure. Patients with G6PD deficiency (an enzyme used to maintain the stability of red blood cell membranes) were found to be at risk of hemolysis following a high dose administration of vitamin C. Appropriate precautions should thus be taken for patients with the above case histories before treatment.
Anti-cancer mechanisms of vitamin C. Vitamin C is synthesized from glucose in four metabolic steps in most animals (16 ). The molecular shape of vitamin C and its oxidized form DHA is remarkably similar to glucose ( Figure 4). Cancer cells often mistake vitamin C for glucose and demonstrate enhanced uptake of Vitamin C by active transport using glucose receptors (GLUT1). Also since cancer cells require a large amount of glucose to sustain their high energy requirements for continuous proliferation and expansion, a large amount of vitamin C if presented to cancer cells will be absorbed by them in excess of that absorbed by normal cells. Once the vitamin C molecule is internalized by its receptors, it generates the superoxide ion in a reaction requiring the presence of iron and copper. This superoxide free radical is converted into hydrogen peroxide which is capable of inducing extensive cellular damage. In normal cells the anti-oxidant enzyme catalase converts the hydrogen peroxide into water and oxygen. In cancer cells, however, since the enzyme catalase is present in very small amounts the hydrogen peroxide is instead converted to even more reactive hydroxyl radicals in a reaction requiring iron and copper (26) (Figure 6). An increase in the oxidative stress in malignant cells consequently generates reactive oxygen species that cannot be eliminated and result in cancer cell death.
Since normal cells have protein-bound iron and no hydrogen peroxide, vitamin C cannot increase oxidative stress in these cells. Actually, in normal cells the only effect of vitamin C is decreased oxidative stress. Additionally, vitamin C in sufficient amounts prevents the accumulation of hydrogen peroxide thus preventing the cells from oxidative damage and transformation into malignant cells.
Therefore, it can be inferred that due to the differences in cellular metabolism between cancer and normal cells, Vitamin C selectively inhibits the former while maintains and strengthens the latter (26). Chemotherapy on the other hand, has adverse effects on both normal and cancerous cells. In cancer cells the increased state of intracellular oxidative stress is exacerbated by the additional pro-oxidant effects of chemotherapeutic agents causing cell death.
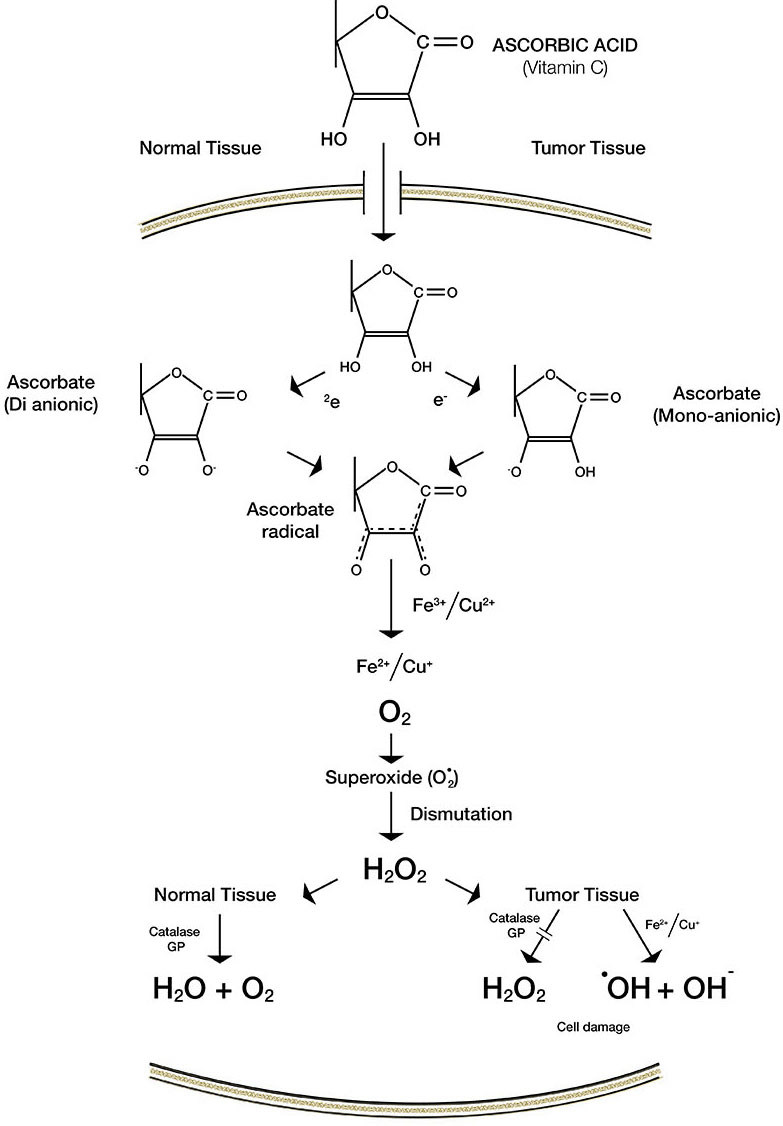
Figure 6. Difference in the mechanism of action of Vitamin C in normal versus tumor tissue.
Studies show that vitamin C also elicits caspase-mediated apoptosis (57, 58) and autophagy (63) in a variety of cancer cell types. Vitamin C inhibits hyaluronidase, an enzyme that tumors use to metastasize and invade distal organs throughout the body. In addition, upon treatment with Vitamin C depletion of cellular ATP levels was found in many cancer models. Downstream from DNA damage and ATP depletion, ZTM/AMPK signaling is activated which in turn results in tumor growth inhibition by cell cycle arrest, apoptosis, necrosis and autophagy. Vitamin C also has inhibitory effect on hypoxia-inducible factor 1⍺ (HIF-1⍺) (64) which regulates glucose metabolism and promotes oncogenic processes in cancer cells.
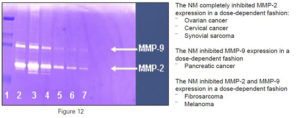
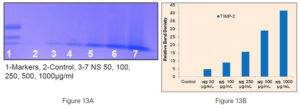
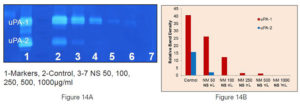
Figure 7. Gel zymographs and densitometric analysis.
A) Inhibition of MMP2 and MMP9 secretion in fibrosarcoma HT-1080 cells;
B) Dose dependent increase in expression of tissue inhibitors of matrix metalloproteinases (TIMP-2) in prostate cancer cells DU-145;
C) Dose dependent inhibition of expression of u-PA in prostate cancer cells DU-145 by increasing concentrations of NM – Lane 1: Marker, Lane 2: Control, Lane 3-7: NM,50, 100, 250, 500, 1000µg/ml
Vitamin C in combination with micronutrients. The majority of studies investigating anti-cancer effects of micronutrients used individual compounds or just a combination of a few (65, 66, 67, 68).
In our anti-cancer approach we targeted key mechanisms essential in facilitating cancer cell growth, invasion and angiogenesis by using a specifically selected combination of micronutrients. It included vitamin C, lysine, proline, green tea extract, and other nutrients essential in inhibiting oxidative stress and facilitating a natural barrier around cancer cells by supporting the synthesis of extracellular matrix and inhibiting its proteolytic degradation. Due to the synergistic action of a variety of anti-carcinogenic components, the micronutrient mixture (NM) was found to be effective in controlling cancer cell proliferation, invasion, metastasis and angiogenesis. Our studies clearly indicate that NM could inhibit cell proliferation in over 50 human cancer cell types selected on the basis of organ malignancies (carcinomas, sarcomas and leukemia) (69). These in vitro findings were also confirmed by in vivo studies. NM inhibited growth of cancer cells implanted as xenografts in athymic mice. It also inhibited cancer development induced by chemicals, such as N-methyl N-Nitrosourea (MNU) in the mammary glands of rats, Urethane in mice lung cancer and dimethyl ben-anthracene (DMBA) in skin cancer (69).
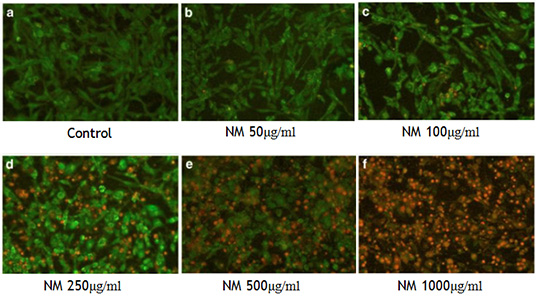
Figure 8. NM induces apoptosis in rhabdomyosarcoma cells. (Live Green Caspase detection)
The NM also inhibited invasion of several human cancer cell lines in a dose dependent fashion. For instance, NM inhibited metastasis of B16FO melanoma cells into lungs (70) from spleen into liver (71), and from testicles to lungs (72), in a mouse model. The inhibition of invasion by NM can be attributed to its inhibitory effect on the synthesis and activity of MMPs, the enzymes responsible for digestion of the extracellular cells. MMPs are elevated in several types of human cancers and are associated with a poor prognosis. Thus by inhibiting the MMP activity of several different cancer cell lines (Figure 7), NM inhibits not just multiplication but also spread of cancer (73). In addition, was effective in inducing cell death in all tested cancer cell types (74) (Figure 8).
Our results suggest that a combination of vitamin C and other micronutrients can therefore be effective in a variety of ways by utilizing and targeting mechanisms specific to cancer cells. The NM offers a multi-targeted non-toxic approach to fighting cancer without a need for mega doses of micronutrients and provides a therapeutic alternative for the extremely toxic current cancer treatments.
References
- Cairn J. The origin of human cancer. 1987. 289: 353-357.
- DeVita V.T. The evolution of therapeutic research in cancer. N Engl J Med. 1978; 298 (16): 907-10.
- Pecorino L. Molecular Biology of Cancer: Mechanisms, Targets and Therapeutics. Oxford University Press; 2005.
- Hanahan D, Weinberger RA. The hallmarks of cancer. 2000; 100 (1): 57-70.
- Norum KR, Grav HJ. Axel Holst and Theodor Frolich–pioneers in the combat of scurvy. Tidsskr Nor Laegeforen, 2002; 122(17): 1686-1687.
- Carpenter KJ. The history of scurvy and vitamin C. Cambridge, Cambridge Univ. Press. 8: pp VIII, 288; 1986.
- Lind J. A treatise of scurvy. Edinburgh: Sands, Murray & Cochran; 1753.
- Svirbely J, Szent-Gyorgyi A. Hexuronic acid as antiscorbutic factor. 1932; 129: 576 -576.
- McCormick WJ. Ascorbic acid as a chemotherapeutic agent. Arch Pediat. 1952; 69: 151-155.
- Sauberlich HE. Pharmacology of Vitamin C. Annu Rev Nutr. 1994; 14: 371-391.
- Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutr J. 2003; 2:7, 1-10.
- Gaby SK, Singh VN. Vitamin C. Vitamin intake and health. A Scientific Review. New York. NY: Marcel Dekker; 1991: 105-108.
- Bates CJ. Bioavailability of vitamin C. Eur J Clin Nutr. 1997; 51 Suppl 1: 28-33.
- Mangels AR, Block G, Frey CM, Patterson BH, Taylor PR, Norkus EP. The bioavailability to human of ascorbic acid from orange juice and cooked broccoli is similar to that of synthetic ascorbic acid. J Nutr. 1993; 123: 1054-1061.
- Gregory JF. Ascorbic acid bioavailability in food and supplements. Nutr Rev. 1993; 51: 301-303.
- Burns JJ. Biosynthesis of L-ascorbic acid: basic defect in scurvy. Am J Med. 1959; 26: 740.
- Vera JC, Rivas CI, Fitchburg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. 1993; 364: 79-82.
- Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr. 2005; 25: 105-125.
- Agus DB, Gambler SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, Golde DW. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997; 100 (11): 2842-2848.
- Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. 1999; 399 (6731): 70-75.
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. New Engl J Med. 1986; 31: 892-902.
- Bendich A. Antioxidant micronutrients and immune responses. In: Bendich A, Chandra RK, ed. Micronutrients and immune functions. Ann N Y Acad Sci. 1999; 587: 168-80.
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA. 1989; 86: 6377-6381.
- Frei B. Reactive oxygen species and antioxidant vitamins: Mechanism of action. Am J Med. 1994; 97: 5S-13S.
- Berg R.A., Kerr. J.S. (1992) Nutritional aspects of collagen metabolism. Annu Rev Nutr. 12: 369-390.
- Gonzalez MJ, Miranda-Massari JR. Springer briefs in Cancer Research: New Insights on Vitamin C and Cancer. New York: NY Springer; 2014.
- Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991; 53: 270S-282S.
- Lee KW, Lee HJ, Surh YJ, Lee CY. Vitamin C and cancer chemoprevention: reappraisal. Am J Clin Nutr. 2003; 78: 1074-1078.
- Enstrom EJ, Kanim LE, Klein MA. Vitamin C intake and mortality among a sample of the United States population. 1992; 3: 194-202.
- World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition and prevention of cancer: a global perspective. (1997) Washington, DC. AICR
- Holman RA. A method of destroying a malignant rat tumor in vivo. Nature. 1957; 179: 1033.
- Leung PY, Miyashita K, Young M, Tsao CS. Cytotoxic effect of ascorbate and its derivatives on cultured malignant and nonmalignant cell lines. Anticancer Res. 1993; 13: 47-80.
- Park CH, Amare M, Savin MA, Hoogstraten B. Growth suppression of human leukemia cells in vitro by L-ascorbic acid. Cancer Res. 1980; 40: 1062-1065.
- Bishun NP, William DC, Basu TK, Metcalfe S. The effect of ascorbic acid on RNA and protein synthesis on two cultured cell lines in vitro. 1979: 25: 29-36.
- Bishun NP, Basu TK, Metcalfe S, Williams DC. The effect of ascorbic acid (vitamin C) on two tumor cell lines in culture. 1978; 35: 160-162.
- Bordignon B, Chiron J, Fontes M. Ascorbic acid derivatives as a new class of antiproliferative molecules. Cancer Lett. 2013; 338(2): 317-327.
- Park S, Han SS, Park CH, et al. L-ascorbic acid induces apoptosis in acute myeloid leukemia cells via hydrogen peroxide-mediated mechanisms. Int J Biochem Cell Biol. 2004; 36: 2180-2195.
- Park S, Lee J, Yeom C.H. A proteomic approach to the identification of early molecular targets changed by L-ascorbic acid in NB4 human leukemia cell. Cell Biochem. 2006; 99: 1628-1641.
- Park S. The effect of high concentrations of vitamin C on cancer cells. 2013; 5: 3496-3505.
- Roomi MW, House D, Eckert-Maksic M, Maksic ZB, Tsao CS. (1998) Growth suppression of malignant leukemia cell line in vitro by ascorbic acid (vitamin C) and its derivatives. Cancer Letter. 1998; 122: 93-99.
- Naidu AK, Wiranowska M, Kori SH, Prockop LD, Kulkarni AP. Inhibition of human glioma cell proliferation and glutathione-S-transferase by ascorbyl esters and interferon. Anticancer Res. 1993; 13 (5A): 1469-1475.
- Chen Q, Espey MG, Krishna MC, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005; 102(38): 13604-13609.
- Chen Q, Polireddy K, Chen P, Dong R. The unpaved journey of vitamin C in cancer treatment. Can J Physiol Pharmacol. 2015; 93 (12): 1055-63.
- Cameron E, Pauling E, Leibovitz B. Ascorbic acid and cancer: a review. Cancer Res. 1979; 39: 663-681.
- Tsao CS. Inhibiting effect of ascorbic acid on the growth of human mammary tumor xenografts. Am J Clin Nutr. 1991; 54 (6 Suppl): 1274S-1280S.
- Chen Q, Espey MG, Sun AY, et al. (2008) Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA. 2008; 105 (32): 11105-11109.
- Liehr JG. Vitamin C reduces the incidence and severity of renal tumors induced by estradiol or diethylstibesterol. Am J Clin Nutr. 1991; 54 (6 Suppl): 1256S-1260S.
- Cha J, Roomi MW, Kalinovsky T, Niezwiecki A, Rath M. Ascorbate supplementation inhibits growth and metastasis of B16F0 melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int J Oncol. 2013; 42 (1): 55-64.
- Yun J, Mullark E. Lu, C. et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. 2015. DOI: 10.1126/science.aaa5004
- Cameron E, Campbell A. The orthomolecular treatment of cancer. Clinical trial of high dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact. 1974; 9 (4): 285-315.
- Cameron E, Pauling L. Supplemental ascorbate in supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1976, 73 (10): 3685-3689.
- Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1978; 75 (9): 4538-4542.
- Murata A, Morishige F, Yamaguchi H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int J Vitam Nutr Res Suppl. 1982; 23: 101-113.
- Ohno S, Ohno Y, Suzuki N, Soma G, Inoue M. High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. AntiCancer Res. 2009; 29(3): 809-815.
- Hoffer LJ, Levine M, Assouline S, et al. (2008) Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008; 19 (11): 1969-1974.
- Moertel CG, Fleming TR, Creagan ET, Robin J, O’Connell MJ, Ames MM. High dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double–blind comparison. N Engl J Med. 1985; 312 (3): 137-141.
- Hong SW, Jin DH, Hahm ES, et al. Ascorbate (vitamin C) induces cell death through the apoptosis-inducing factor in human breast cancer cells. Oncol Rep. 2007; 18(4): 811-815.
- Kang JS, Cho D, Kim Y, et al. L-ascorbic acid (vitamin C) induces the apoptosis of B16 murine melanoma cells via a caspase-8-independent pathway. Cancer Immunol Immunother. 2003; 52 (11): 693-698.
- Padayatty SJ, Riordan HD, Hewitt SM, Katz A., Hoffer LJ, Levine M. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ. 2006; 174: 937-942.
- Riordan NH, Riordan HD, Casciari JP. Clinical and experimental experiences with intravenous vitamin C. J Ortho Med. 2000, 15 (4): 201-213.
- Riordan NH, Jackson JA, Riordan HD. Intravenous vitamin C in a terminal cancer patient. J Ortho Med. 1996; 11 (2): 80-82.
- Riordan NH, Riordan HD, Meng X, Li Y, Jackson JA. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses. 1995; 44 (3): 207-213.
- Cullen JJ. Ascorbate induces autophagy in pancreatic cancer. Autophagy. 2010; 6(3): 421-422.
- Kawada H, Kaneko M, Sawanobori M, et al. High concentrations of L-ascorbic acid specifically inhibit the growth of human leukemic cells via downregulation of HIF-1⍺ PLOS One. 2013; 8(4): e62717.
- Demeule M, Brossard M, Page M, Gingras D, Beliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochem Biophy Acta. 2000; 1478 (1): 51-60.
- Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000; 71 (6 Suppl): 1698s-1702s.
- Kawakami S, Kageyama Y, Fujii Y, Kihara K, Oshima H. Inhibitory effect of N-acetylcysteine on invasion and MMP-9 production of T24 human bladder cancer cells. Anticancer Res. 2001; 21 (1A): 213-219.
- Yoon SO, Kim MM, Chung AS. Inhibitory effect of selenite on invasion of HT-1080 tumor cells. J Biol Chem. 2001; 276 (23): 20085-20092.
- Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Micronutrient synergy—a new tool in effective control of metastasis and other key mechanisms of cancer. Cancer Metastasis Rev. 2010; 29 (3): 529-542.
- Roomi MW, Roomi N, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Inhibition of pulmonary metastasis of melanoma B16FO cells in C57BL/6 mice by a nutrient mixture consisting of ascorbic acid, lysine, proline , arginine and green tea extract. Exp Lung Res. 2006; 32 (10): 517-530.
- Roomi MW, Kalinovsky T, Roomi NW, Ivanov V, Rath M, Niedzwiecki A. Suppression of growth and hepatic metastasis of murine B16FO melanoma cells by a novel nutrient mixture. Oncol Rep. 2008; 20 (4): 809-817.
- Roomi MW, Kalinovsky T., Roomi NW, Niedzwiecki A, Rath M. Suppression of metastasis of intratesticular inoculation of B16FO melanoma cells by a novel nutrient mixture in male athymic nude mice, Exp Ther Med. 2012, 4 (5): 775-780.
- Roomi MW, Bhanap B, Niedzwiecki A, Rath M. (2015) Suppression of matrix metalloproteinases -2 and -9 in various human cancer cell lines by a nutrient mixture. J Oncobiomarkers. 2015; 2 (2): 1-17
- Roomi MW, Shanker N, Niedzwiecki A, Rath M. (2015) Induction of apoptosis in the human prostate cancer cell line DU-145 by a novel micronutrient formulation. OJApo. 2015; 4: 11-21.
- Cairn J. The origin of human cancer. 1987. 289: 353-357.
- DeVita V.T. The evolution of therapeutic research in cancer. N Engl J Med. 1978; 298 (16): 907-10.
- Pecorino L. Molecular Biology of Cancer: Mechanisms, Targets and Therapeutics. Oxford University Press; 2005.
- Hanahan D, Weinberger RA. The hallmarks of cancer. 2000; 100 (1): 57-70.
- Norum KR, Grav HJ. Axel Holst and Theodor Frolich–pioneers in the combat of scurvy. Tidsskr Nor Laegeforen, 2002; 122(17): 1686-1687.
- Carpenter KJ. The history of scurvy and vitamin C. Cambridge, Cambridge Univ. Press. 8: pp VIII, 288; 1986.
- Lind J. A treatise of scurvy. Edinburgh: Sands, Murray & Cochran; 1753.
- Svirbely J, Szent-Gyorgyi A. Hexuronic acid as antiscorbutic factor. 1932; 129: 576 -576.
- McCormick WJ. Ascorbic acid as a chemotherapeutic agent. Arch Pediat. 1952; 69: 151-155.
- Sauberlich HE. Pharmacology of Vitamin C. Annu Rev Nutr. 1994; 14: 371-391.
- Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutr J. 2003; 2:7, 1-10.
- Gaby SK, Singh VN. Vitamin C. Vitamin intake and health. A Scientific Review. New York. NY: Marcel Dekker; 1991: 105-108.
- Bates CJ. Bioavailability of vitamin C. Eur J Clin Nutr. 1997; 51 Suppl 1: 28-33.
- Mangels AR, Block G, Frey CM, Patterson BH, Taylor PR, Norkus EP. The bioavailability to human of ascorbic acid from orange juice and cooked broccoli is similar to that of synthetic ascorbic acid. J Nutr. 1993; 123: 1054-1061.
- Gregory JF. Ascorbic acid bioavailability in food and supplements. Nutr Rev. 1993; 51: 301-303.
- Burns JJ. Biosynthesis of L-ascorbic acid: basic defect in scurvy. Am J Med. 1959; 26: 740.
- Vera JC, Rivas CI, Fitchburg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. 1993; 364: 79-82.
- Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr. 2005; 25: 105-125.
- Agus DB, Gambler SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, Golde DW. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997; 100 (11): 2842-2848.
- Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. 1999; 399 (6731): 70-75.
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. New Engl J Med. 1986; 31: 892-902.
- Bendich A. Antioxidant micronutrients and immune responses. In: Bendich A, Chandra RK, ed. Micronutrients and immune functions. Ann N Y Acad Sci. 1999; 587: 168-80.
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA. 1989; 86: 6377-6381.
- Frei B. Reactive oxygen species and antioxidant vitamins: Mechanism of action. Am J Med. 1994; 97: 5S-13S.
- Berg R.A., Kerr. J.S. (1992) Nutritional aspects of collagen metabolism. Annu Rev Nutr. 12: 369-390.
- Gonzalez MJ, Miranda-Massari JR. Springer briefs in Cancer Research: New Insights on Vitamin C and Cancer. New York: NY Springer; 2014.
- Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991; 53: 270S-282S.
- Lee KW, Lee HJ, Surh YJ, Lee CY. Vitamin C and cancer chemoprevention: reappraisal. Am J Clin Nutr. 2003; 78: 1074-1078.
- Enstrom EJ, Kanim LE, Klein MA. Vitamin C intake and mortality among a sample of the United States population. 1992; 3: 194-202.
- World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition and prevention of cancer: a global perspective. (1997) Washington, DC. AICR
- Holman RA. A method of destroying a malignant rat tumor in vivo. Nature. 1957; 179: 1033.
- Leung PY, Miyashita K, Young M, Tsao CS. Cytotoxic effect of ascorbate and its derivatives on cultured malignant and nonmalignant cell lines. Anticancer Res. 1993; 13: 47-80.
- Park CH, Amare M, Savin MA, Hoogstraten B. Growth suppression of human leukemia cells in vitro by L-ascorbic acid. Cancer Res. 1980; 40: 1062-1065.
- Bishun NP, William DC, Basu TK, Metcalfe S. The effect of ascorbic acid on RNA and protein synthesis on two cultured cell lines in vitro. 1979: 25: 29-36.
- Bishun NP, Basu TK, Metcalfe S, Williams DC. The effect of ascorbic acid (vitamin C) on two tumor cell lines in culture. 1978; 35: 160-162.
- Bordignon B, Chiron J, Fontes M. Ascorbic acid derivatives as a new class of antiproliferative molecules. Cancer Lett. 2013; 338(2): 317-327.
- Park S, Han SS, Park CH, et al. L-ascorbic acid induces apoptosis in acute myeloid leukemia cells via hydrogen peroxide-mediated mechanisms. Int J Biochem Cell Biol. 2004; 36: 2180-2195.
- Park S, Lee J, Yeom C.H. A proteomic approach to the identification of early molecular targets changed by L-ascorbic acid in NB4 human leukemia cell. Cell Biochem. 2006; 99: 1628-1641.
- Park S. The effect of high concentrations of vitamin C on cancer cells. 2013; 5: 3496-3505.
- Roomi MW, House D, Eckert-Maksic M, Maksic ZB, Tsao CS. (1998) Growth suppression of malignant leukemia cell line in vitro by ascorbic acid (vitamin C) and its derivatives. Cancer Letter. 1998; 122: 93-99.
- Naidu AK, Wiranowska M, Kori SH, Prockop LD, Kulkarni AP. Inhibition of human glioma cell proliferation and glutathione-S-transferase by ascorbyl esters and interferon. Anticancer Res. 1993; 13 (5A): 1469-1475.
- Chen Q, Espey MG, Krishna MC, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005; 102(38): 13604-13609.
- Chen Q, Polireddy K, Chen P, Dong R. The unpaved journey of vitamin C in cancer treatment. Can J Physiol Pharmacol. 2015; 93 (12): 1055-63.
- Cameron E, Pauling E, Leibovitz B. Ascorbic acid and cancer: a review. Cancer Res. 1979; 39: 663-681.
- Tsao CS. Inhibiting effect of ascorbic acid on the growth of human mammary tumor xenografts. Am J Clin Nutr. 1991; 54 (6 Suppl): 1274S-1280S.
- Chen Q, Espey MG, Sun AY, et al. (2008) Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA. 2008; 105 (32): 11105-11109.
- Liehr JG. Vitamin C reduces the incidence and severity of renal tumors induced by estradiol or diethylstibesterol. Am J Clin Nutr. 1991; 54 (6 Suppl): 1256S-1260S.
- Cha J, Roomi MW, Kalinovsky T, Niezwiecki A, Rath M. Ascorbate supplementation inhibits growth and metastasis of B16F0 melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int J Oncol. 2013; 42 (1): 55-64.
- Yun J, Mullark E. Lu, C. et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. 2015. DOI: 10.1126/science.aaa5004
- Cameron E, Campbell A. The orthomolecular treatment of cancer. Clinical trial of high dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact. 1974; 9 (4): 285-315.
- Cameron E, Pauling L. Supplemental ascorbate in supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1976, 73 (10): 3685-3689.
- Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1978; 75 (9): 4538-4542.
- Murata A, Morishige F, Yamaguchi H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int J Vitam Nutr Res Suppl. 1982; 23: 101-113.
- Ohno S, Ohno Y, Suzuki N, Soma G, Inoue M. High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. AntiCancer Res. 2009; 29(3): 809-815.
- Hoffer LJ, Levine M, Assouline S, et al. (2008) Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008; 19 (11): 1969-1974.
- Moertel CG, Fleming TR, Creagan ET, Robin J, O’Connell MJ, Ames MM. High dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double–blind comparison. N Engl J Med. 1985; 312 (3): 137-141.
- Hong SW, Jin DH, Hahm ES, et al. Ascorbate (vitamin C) induces cell death through the apoptosis-inducing factor in human breast cancer cells. Oncol Rep. 2007; 18(4): 811-815.
- Kang JS, Cho D, Kim Y, et al. L-ascorbic acid (vitamin C) induces the apoptosis of B16 murine melanoma cells via a caspase-8-independent pathway. Cancer Immunol Immunother. 2003; 52 (11): 693-698.
- Padayatty SJ, Riordan HD, Hewitt SM, Katz A., Hoffer LJ, Levine M. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ. 2006; 174: 937-942.
- Riordan NH, Riordan HD, Casciari JP. Clinical and experimental experiences with intravenous vitamin C. J Ortho Med. 2000, 15 (4): 201-213.
- Riordan NH, Jackson JA, Riordan HD. Intravenous vitamin C in a terminal cancer patient. J Ortho Med. 1996; 11 (2): 80-82.
- Riordan NH, Riordan HD, Meng X, Li Y, Jackson JA. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses. 1995; 44 (3): 207-213.
- Cullen JJ. Ascorbate induces autophagy in pancreatic cancer. Autophagy. 2010; 6(3): 421-422.
- Kawada H, Kaneko M, Sawanobori M, et al. High concentrations of L-ascorbic acid specifically inhibit the growth of human leukemic cells via downregulation of HIF-1⍺ PLOS One. 2013; 8(4): e62717.
- Demeule M, Brossard M, Page M, Gingras D, Beliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochem Biophy Acta. 2000; 1478 (1): 51-60.
- Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000; 71 (6 Suppl): 1698s-1702s.
- Kawakami S, Kageyama Y, Fujii Y, Kihara K, Oshima H. Inhibitory effect of N-acetylcysteine on invasion and MMP-9 production of T24 human bladder cancer cells. Anticancer Res. 2001; 21 (1A): 213-219.
- Yoon SO, Kim MM, Chung AS. Inhibitory effect of selenite on invasion of HT-1080 tumor cells. J Biol Chem. 2001; 276 (23): 20085-20092.
- Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Micronutrient synergy—a new tool in effective control of metastasis and other key mechanisms of cancer. Cancer Metastasis Rev. 2010; 29 (3): 529-542.
- Roomi MW, Roomi N, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Inhibition of pulmonary metastasis of melanoma B16FO cells in C57BL/6 mice by a nutrient mixture consisting of ascorbic acid, lysine, proline , arginine and green tea extract. Exp Lung Res. 2006; 32 (10): 517-530.
- Roomi MW, Kalinovsky T, Roomi NW, Ivanov V, Rath M, Niedzwiecki A. Suppression of growth and hepatic metastasis of murine B16FO melanoma cells by a novel nutrient mixture. Oncol Rep. 2008; 20 (4): 809-817.
- Roomi MW, Kalinovsky T., Roomi NW, Niedzwiecki A, Rath M. Suppression of metastasis of intratesticular inoculation of B16FO melanoma cells by a novel nutrient mixture in male athymic nude mice, Exp Ther Med. 2012, 4 (5): 775-780.
- Roomi MW, Bhanap B, Niedzwiecki A, Rath M. (2015) Suppression of matrix metalloproteinases -2 and -9 in various human cancer cell lines by a nutrient mixture. J Oncobiomarkers. 2015; 2 (2): 1-17
- Roomi MW, Shanker N, Niedzwiecki A, Rath M. (2015) Induction of apoptosis in the human prostate cancer cell line DU-145 by a novel micronutrient formulation. OJApo. 2015; 4: 11-21.