Seaweed – a substitute for ascorbic acid
Objective
Seaweeds are an abundant and readily available source of both bulk nutrients and biologically active nutrients. We hypothesized that seaweed polysaccharide fucoidan could serve as a temporary substitute for ascorbic acid under conditions of vitamin C deficiency by beneficially affecting structural properties of the arterial wall.
Methods
This was tested in an experimental model of cultured smooth muscle cells (AoSMC) and endothelial cells (AoEC) isolated from human aorta and cultured dermal fibroblasts (DFB) isolated from human skin. The effects of fucoidan in cultured cells were characterized by immunochemical assessment of deposition of selected extracellular matrix (ECM) proteins and glycosaminoglycans.
Results
Physiological concentrations of fucoidan effectively stimulated ECM deposition of proteins, presented by collagen types I and IV, and glycosaminoglycans, presented by heparan sulfate and hyaluronic acid, by AoSMC and DFB in manner and extent comparable to corresponding actions of ascorbic acid. Activity of a combination of two nutrients did not exceed activity of the compounds applied individually. Neither fucoidan nor ascorbic acid modulated ECM components deposition by AoEC under used experimental conditions. Structural characteristics of ECM components deposited by cultured cells under influence of fucoidan remain a subject of future research.
Conclusion
The results support our initial hypothesis on a capacity of seaweed sulfated polysaccharide fucoidan to possess vitamin C-like activity on ECM components production and deposition by arterial wall resident cells.
Keywords
Seaweed;
Fucoidan;
Smooth muscle cells;
ECM;
Collagen I;
Collagen IV;
Heparan sulfate;
Hyaluronic acid;
Glycosaminoglycans;
Ascorbate;
Vitamin C.
Correspondence to
Dr. Aleksandra Niedzwiecki
Dr. Rath Research Institute
1260 Memorex Drive
Santa Clara
CA 95050
USA
Email: author@jcmnh.org
Introduction
Prevalence of CVD. Cardiovascular disease (CVD) became a leading cause of human mortality and morbidity in the modern world [1]. Major manifestations of CVD, such as atherosclerosis of the arterial wall, myocardial infarction, heart failure, stroke, hypertension, arrhythmia, and peripheral vascular disease, are direct results of poor structural stability and function of the arterial wall [2].
Cellular and extracellular composition of the arterial wall. The structure of the arterial wall consists of two major components: arterial wall resident cells and extracellular matrix (ECM) produced and deposited by the resident cells [as reviewed in 3]. Overall function of the cardiovascular system depends on preserving a healthy environment for resident cells of the arterial wall and structural stability of cell-produced ECM. Resident arterial cells are presented by endothelial cells, smooth muscle cells and fibroblasts. Arterial endothelial cells line the luminal surface of the artery by forming a one-cell thick continuous cellular layer throughout the entire arterial tree. Their primary function is proper maintenance of the blood-tissue barrier. They regulate transport of nutrients, biological regulators and leucocytes to inner layers of the arterial wall. They also produce and deposit components of underlying basement membrane – a layer of extracellular matrix which separates the endothelial layer from inner layers of the arterial wall, provide mechanical support for endothelial cells and serve an important role in endothelial layer barrier function.
Smooth muscle cells occupy the inner, most prominent part of the arterial wall. Their primary function is contraction and relaxation in response to mechanical stress caused by blood flow pulses from the contracting heart. They also produce and deposit ECM components to build a three-dimensional mesh network which provides structural support for the entire arterial wall.
Fibroblasts and produced by them ECM form the outer layer of the arterial wall – the so-called adventitia. Its function is protecting integrity of the arterial wall within the environment of the numerous tissues and organs in the body.
Extracellular matrix inside the arterial wall is an example of connective tissue – a building material for all tissues and organs of the human body. ECM composition is very complex and it is tailored to specific requirements of different tissues and organs. Generally, the main ECM components are proteins and complex polymeric carbohydrates belonging to a class of glycosaminoglycans (GAGs).
The most prominent ECM proteins are collagens [4]. There are more than thirty reported types of collagens serving different structural and functional roles in arterial wall ECM. The most important are the type I and type IV collagens. The ratio of the contents of these two collagen types seems to be specific for different tissues and it seems to be modulated during pathological conditions of disease development [5].
The majority of GAGs in extracellular matrix of the arterial wall are covalently bound to ECM proteins forming so-called proteoglycans [6]. A notable example of GAGs covalently bound to proteins is heparan sulfate, which provides binding sites for numerous biological regulating factors within the arterial wall. Notable exception to this rule is a glycosaminoglycan called hyaluronic acid which attaches to ECM proteins by non-covalent binding [7]. Hyaluronic acid serves an important role as an interconnector between different ECM components and also between ECM and arterial wall resident cells. Hyaluronic acid also plays a key role in regulation of mechanical properties of the arterial wall through its capacity to retain water molecules and therefore regulate intra-tissue osmotic pressure.
Improper function of resident arterial wall cells and compromised composition of extracellular matrix within the wall ultimately result in the development of CVD.
Importance of natural nutrients. So far, decades long attempts to treat CVD with synthetic medications aimed at different pathogenic disease manifestations did not bring an effective overall control over the disease frequency, morbidity or mortality. Extensive use of beta-blockers, statins, diuretics, and others, merely address symptomatic manifestations of CVD and offer only modest reduction in severity of patient suffering and life span extension [8].
More success has been achieved with preventive measures such as introduction of a healthy lifestyle which includes the avoidance of tobacco, regular moderate physical exercise and dietary modifications [9]. Dietary approaches include introduction and regular consumption of foods rich in natural biologically active and/or essential nutrients [10].
Key role of ascorbate in building and maintaining proper structure of the arterial wall. Brief insight into scurvy and scurvy-CVD connection. Humans and a few animal species including apes, guinea pigs and several fruit-eating bats are lacking a capacity for internal synthesis of ascorbic acid, also known as vitamin C. For this reason humans are dependent on getting ascorbic acid from nutritional sources [11]. Ascorbic acid is an essential factor for three key enzymes responsible for proper collagen chain assembly by catalysing hydroxylation of lysine and proline residues of the collagen amino acid primary structure [11]. Acute deficiency in the nutritional ascorbic acid supply causes a development of the “sailor’s disease”, scurvy, in a matter of a few months. The major manifestation of scurvy is a gradual weakening of the mechanical structure of the arterial wall to the point when it fails to keep blood within the blood vessel leading to hemorrhages throughout the body [11].
About a quarter of a century ago Dr. Rath and Dr. Pauling hypothesized that a chronic sub-acute deficiency of ascorbic acid could be a leading cause for gradual development of CVD [12]. This concept addresses different aspects of complex pathogenesis of atherosclerosis and resulting CVD in humans regarding them as a body adaptive response to chronic mechanical weakness of the arterial wall. Many postulates of this concept have found experimental confirmations in more recent studies [13].
When taking in evolutional aspects of human biology, this new concept argues that a development of so-called lipoprotein(a), a unique sticky protein found only in humans and apes, was because of its capacity to temporarily maintain a mechanical stability of the arterial wall during episodes of complete absence or low levels of nutritional supplementation of ascorbic acid. In these terms lipoprotein(a) is serving as a temporary substitute for vitamin C. This postulate does not deny a possibility of existence of other substitutes for vitamin C either inside the body or as nutritional supplements.
Nutritional role of seafood in general and seaweeds in particular. In general seafood is being regarded as a dietary component associated with a “healthy” lifestyle. Notable examples are the so-called Mediterranean diet and a traditional Japanese diet. Seaweeds are regarded as an important component of a healthy diet and as a good nutritional source of Vitamin D, organic forms of iodine and long chain n-3 fatty acids [14]. Other components of seaweeds are being investigated as possible sources of biologically active compounds. Fucoidan, sulfated polysaccharide found in the cell walls of certain seaweeds, has recently been demonstrated to possess different biological activities, including possible effects on cellular production of ECM components [15]. As seaweeds were an abundant and easy-to-reach supply along sea shores for early man during the Stone Age era, they served as an important source of both bulk nutrients and nutritional biologically active regulators.
Hypothesis on the evolutional role of seaweeds as a substitute for ascorbate and goal of the study. We hypothesized that the seaweed component fucoidan could beneficially affect a production of ECM components by human cells and therefore serve as a temporary substitute for vitamin C.
The goal of the study was to investigate the effects of fucoidan on ECM formation by different arterial wall cell types. We used cultured in vitro smooth muscle cells (AoSM) and endothelial cells (AoEC) isolated from human aortas as experimental models for corresponding resident cell types of the human arterial wall. Cultured dermal fibroblasts (DFB) isolated from human skin were used as an experimental model for resident fibroblasts in the adventitia layer of the human arterial wall. ECM deposition was characterized by cell deposition of two major ECM proteins, collagen types I and IV, and two major ECM glycosaminoglycans, heparan sulfate and hyaluronic acid.
We demonstrated that fucoidan can affect ECM deposition by arterial wall cells in beneficial directions resembling actions of ascorbic acid and could serve a role of a temporary substitute for vitamin C.
Materials and Methods
Reagents. All reagents were from Sigma-Aldrich (St. Louis, MO) except where indicated differently.
Cell cultures. Normal human dermal fibroblasts (DFB) were supplied by ATCC (Manassas, VA). Human aortic smooth muscle cells (AoSMC) were purchased from Cambrix (East Rutherford, NJ). Both cell cultures were maintained in DMEM medium (ATCC) containing antibiotics and 5% fetal bovine serum (FBS, ATCC). Human aortic endothelial cells (AoEC) were purchased from Cambrix (East Rutherford, NJ) and maintained in EGM-2 medium (containing 2% FBS) as specified and supplied by Cambrix. All cell cultures were maintained at 37oC and 5% CO2 atmosphere. Cell viability was monitored with MTT assay (Promega). None of the experimental conditions used resulted in statistically significant cell death (data not shown).
ECM components deposition by human cultured cells. For the experiments AoEC, DFB or AoSMC at 5th to 8th passages (two plates per each cell type) were seeded on Collagen type I covered 96 well plastic plates (Becton-Dickinson, collagen I isolated from rat tail tendon) at density 25,000 per square cm and grown to confluence for five to seven days. Tested compounds were added to cells at indicated concentrations for 72 hours in DMEM supplemented with 2% FBS (DF and AoSMC) or in EGM-2 medium (AoEC), and cell-produced extracellular matrix was exposed by sequential treatment with 0.5% Triton X 100 and 20 mM ammonium sulfate in phosphate buffered saline (PBS, Life Technologies) for 3 minutes each at room temperature as described previously [16]. After four washes with PBS, ECM layers were treated with 1% bovine serum albumin (BSA) in PBS for one hour at RT and used immediately.
ECM composition by immunoassay. Immunoasssay was done as described previously [15] by sequential incubation with primary monoclonal antibodies specific to human collagen type I or IV or heparan sulfate (Chemicon) in 1% BSA/PBS for two hours followed by one hour of incubation with secondary goat anti-mouse IgG antibodies labelled with horse radish peroxidase (HRP). For hyaluronic acid assay, a set of biotylated hyaluronic acid-binding protein (US Biological) and Ultra-sensitive streptovidin-HRP (SDT, Baesweiler, Germany) was used. Retained peroxidase activity was measured after the last washing cycle (three times with 0.1% BSA/PBS) using TMB peroxidase substrate reagent (Rockland). Optical density was read with a plate reader (Molecular Devices) at 450 nm and expressed in optical density units.
All plates with cells were treated consecutively in the same day in a strictly timed manner. Therefore, absolute values for HRP retention at ECM covered plates (expressed in optical density units) can be used for direct comparison of individual ECM component deposition by different cell types.
Statistical analysis. Results in figures are means ± SD from three or more repetitions from the most representative out of three independent experiments. Differences between samples were estimated with a two-tailed Student’s t-test using a Microsoft Excel program and were accepted as significant at p levels less than 0.05.
Results
Effects of ascorbic acid and fucoidan on collagen type I deposition into ECM by cultured human arterial wall cells. Experimental results are presented in Figure 1 and results for statistical analysis in Table 1. ECM deposition of collagens by monolayers of cultured cells of different cell types was compared in the presence or absence of tested compounds using immunochemical assays. When cultured in standard cell growth medium, deposition of collagen type I by AoSMC was significantly higher than by DFB or AoEC by 108% and 150%, respectively.
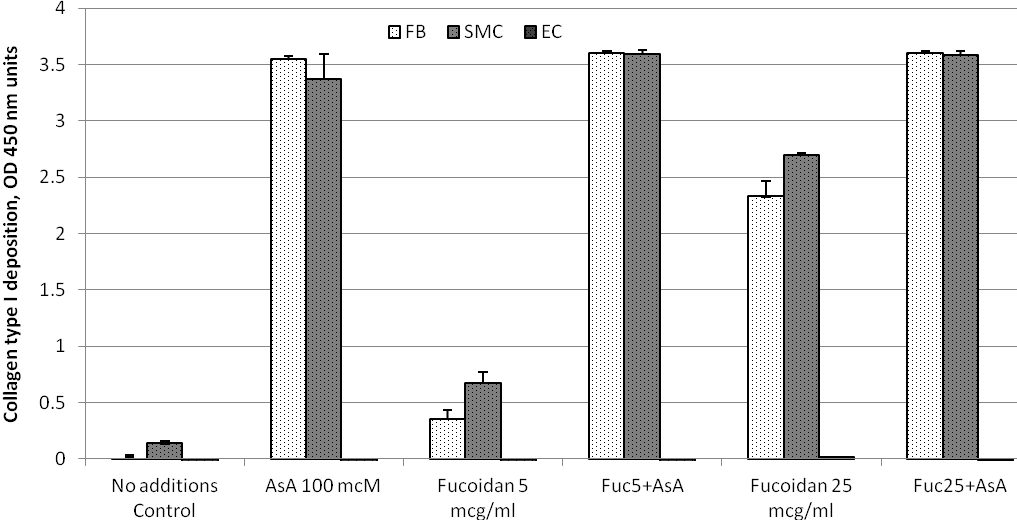
Figure 1. ECM deposition of collagen type I by cultured human arterial wall resident cells: Effects of ascorbic acid and fucoidan. Confluent layers of Aortic Smooth Muscle Cells (AoSMC), dermal fibroblasts (DFB) and Aortic Endothelial cells (AoEC) were supplemented with 5 or 25 mcg/ml fucoidan (Fuc) in the presence or absence of 100 mcM ascorbic acid (AsA) or in plain cell growth medium for 72 hours. Collagen type I content in resulting extracellular matrix (ECM) was estimated by immunochemical assay. All cells were treated identically within the same experiment. For more experimental details refer to Material and Methods section.
Table 1. Statistical analysis of Collagen type I deposition by cultured arterial wall resident cells
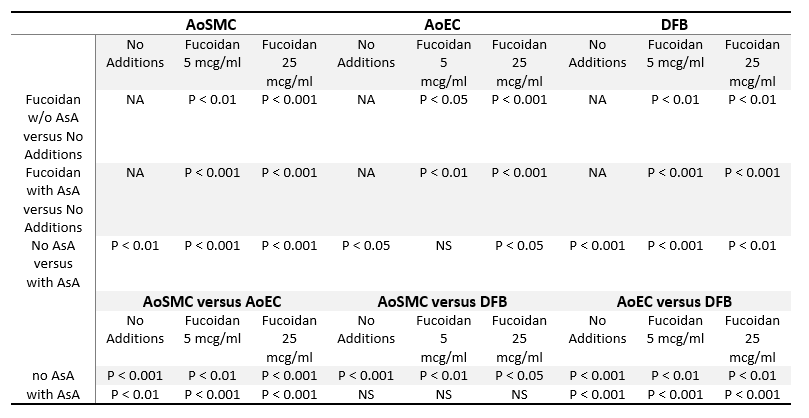
Statistical analysis is shown as p values of Student’s two-tailed t-test for the results presented in Figure 1. Abbreviations: NA, not applicable; NS, non-significant (p=>0.05). Other abbreviations are as in Figure 1 legend.
Addition of 100 mcM ascorbic acid to cell culture medium resulted in dramatic increase of collagen type I content in ECM produced by AoSMC and DFB (13.9 and 30.5 folds, respectively). The difference in final collagen type I ECM content between DFB and AoSMC under influence of ascorbic acid was effectively eliminated by reaching a saturation point of the assay in both cell types. In contrast, ECM deposition of collagen I by AoEC was not changed by ascorbic acid.
Supplementation of cells with fucoidan resulted in dose-dependent increase in ECM collagen I deposition by AoSMC and DFB. At a dose of 25 mcg/ml fucoidan, an increase in ECM collagen I content was 20.3 folds for DFB and 11.24 folds for AoSMC. However, in both cell types it did not reach a saturation point. Collagen type I ECM content in cultured AoEC was not affected by fucoidan supplementation.
Addition of fucoidan to ascorbate-supplemented did not change collagen type I content in any cell type studied.
Effects of ascorbic acid and fucoidan on collagen type IV deposition into ECM by cultured human arterial wall cells. Experimental results are presented in Figure 2 and results for statistical analysis in Table 2. Deposition of collagen type IV by AoEC in the absence of tested compounds was dramatically higher than in HDF and AoSMC (39.7 folds and 19.8 folds, respectively), reaching an assay saturation point. Under these conditions AoSMC produced twice as much collagen type IV than DFB. Addition of 100 mcM ascorbate significantly stimulated collagen IV deposition by DFB and AoSMC (12.9 folds and 3.7 folds, respectively). Ascorbic acid did not modulate collagen IV content in ECM deposited by AoEC. It remained at assay saturation point.
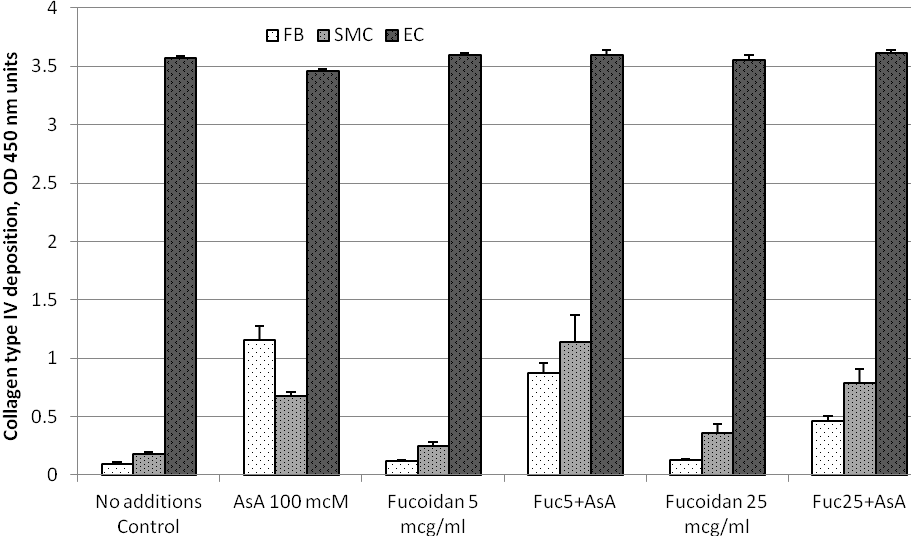
Figure 2. ECM deposition of collagen type IV by cultured human arterial wall resident cells: Effects of ascorbic acid and fucoidan. Experimental details and abbreviations are as in a legend to Figure 1.
Table 2. Statistical analysis of Collagen type IV deposition by cultured arterial wall resident cells
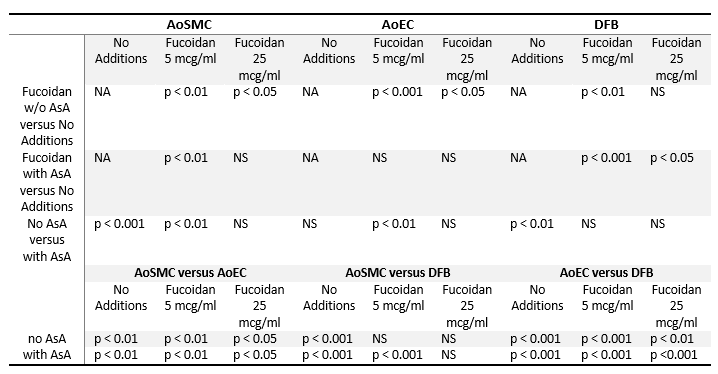
Statistical analysis is shown as p values of Student’s two-tailed t-test for results presented in Figure 2. Abbreviations are as in the notes to Table 1.
Fucoidan caused a significant dose-dependent increase in ECM collagen type IV content both in DFB and AoSMC. However, even at the highest supplementation level tested at 25 mcg/ml fucoidan, collagen IV ECM content did not reach levels of ascorbate dependent effects in both cell types. When used in combination with ascorbic acid, low doses of fucoidan at 5 mcg/ml did not have any significant effects. Higher doses of fucoidan at 25 mcg/ml added together with ascorbate slightly reduced collagen IV ECM content in both DFB and AoSMC cultures. Fucoidan used individually or in combination with ascorbic acid did not affect ECM deposition of collagen type IV in AoEC, as it remained at an assay saturation point.
Effects of ascorbic acid and fucoidan on heparan sulfate deposition into ECM by cultured human arterial wall cells. Experimental results are presented in Figure 3 and results for statistical analysis in Table 3. ECM deposition of heparan sulfate by cells cultured in plain growth medium was significantly higher in DFB as compared to AoSMC and AoEC (6.9 folds and 13.8 folds, respectively) as depicted in Figure 3. Addition of 100 mcM ascorbic acid resulted in further increase in heparan sulfate ECM content in DFB by 90% and in AoSMC by 190 %. Supplementation of cell cultures with fucoidan caused significant increase in heparan sulfate ECM content in DFB which was comparable to the effects of 100 mcM ascorbic acid. The effects of fucoidan on heparan sulfate ECM content in DFB culture were similar at both low and high doses. Combined supplementation of DFB with ascorbic acid and fucoidan did not significantly affect ECM heparan sulfate in comparison with effects of individually tested nutrients. In contrast to DFB, fucoidan stimulated ECM deposition of heparan sulfate in AoSMC in dose-dependent manner both when applied alone or in combination with ascorbic acid. Combination of fucoidan with ascorbic acid was less effective than fucoidan alone in AoSMC. Neither ascorbic acid, fucoidan, or their combination, significantly affected heparan sulfate ECM content in AoEC culture.
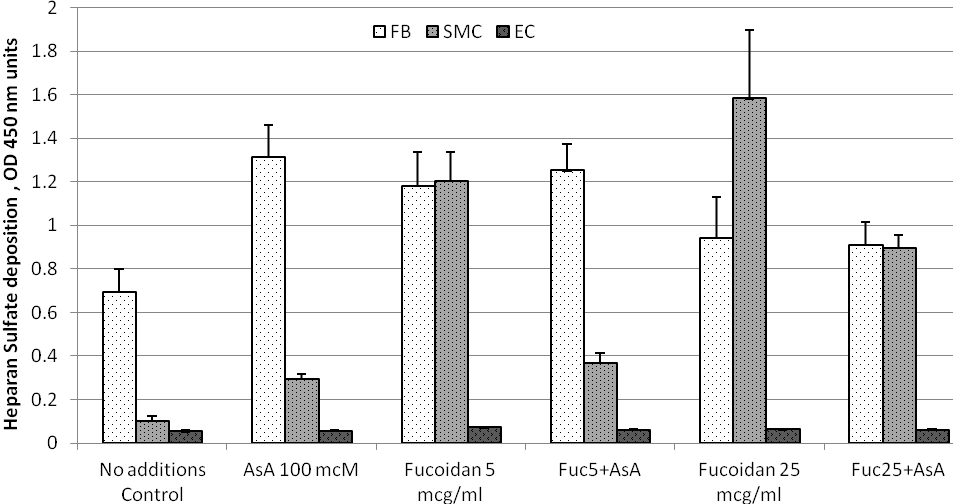
Figure 3. ECM deposition of heparan sulfate by cultured human arterial wall resident cells: Effects of ascorbic acid and fucoidan. Experimental details and abbreviations are as in a legend to Figure 1.
Table 3. Statistical analysis of Heparan Sulfate deposition by cultured arterial wall resident cells

Statistical analysis is shown as p values of Student’s two-tailed t-test for results presented in Figure 3. Abbreviations are as in the notes to Table 1.
Effects of ascorbic acid and fucoidan on hyaluronic acid deposition into ECM by cultured human arterial wall cells. Experimental results are presented in Figure 4 and results for statistical analysis in Table 4. The levels of ECM hyaluronic acid were similar in all three cell types cultured in non-supplemented plain cell growth medium. Ascorbic acid at 100 mcM stimulated levels of ECM hyaluronic acid in DFB by 96% and AoSMC by 45%, but not in AoEC.
Fucoidan did not modulate ECM hyaluronic acid levels in DFB when applied alone and caused a reduction of stimulating effects of ascorbic acid. In AoSMC cultures, fucoidan effectively induced ECM hyaluronic acid only at higher 25 mcg/ml doses. This effect was counteracted by addition of ascorbic acid. Addition of fucoidan to AoEC cultures resulted in reduction of ECM hyaluronic acid at higher doses 25 mcg/ml when used alone and at both low and high doses in the presence of ascorbic acid.
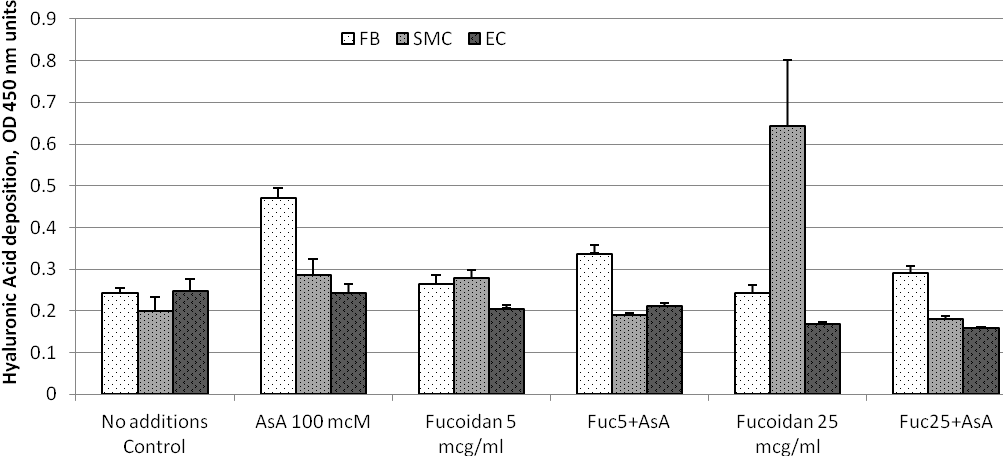
Figure 4. ECM deposition of hyaluronic acid by cultured human arterial wall resident cells: Effects of ascorbic acid and fucoidan. Experimental details and abbreviations are as in a legend to Figure 1.
Table 4. Statistical analysis of Hyaluronic Acid deposition by cultured arterial wall resident cells
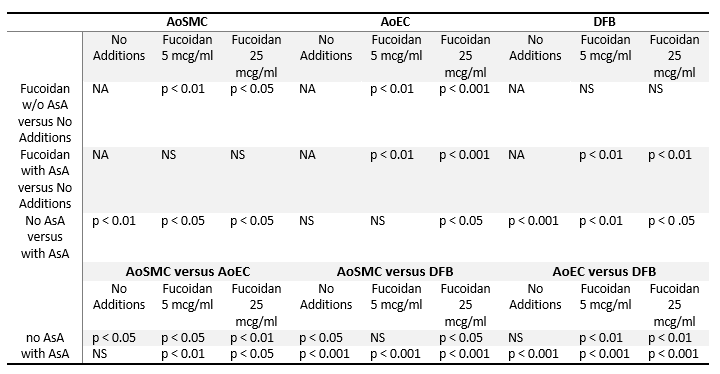
Statistical analysis is shown as p values of Student’s two-tailed t-test for results presented in Figure 4. Abbreviations are as in the notes to Table 1.
Discussion
Stimulating effects of fucoidan on ECM component deposition by arterial cells suggests a possible substitute role in ascorbate deficiency. In this study we have tested whether polysaccharide compound fucoidan isolated from seaweeds could affect deposition of selected ECM components by cultured arterial wall cells and compared its effects to ascorbic acid. Collagens type I and type IV were chosen as typical representatives of ECM proteins in the vascular wall. Heparan sulfate and hyaluronic acid represented ECM associated glycosaminoglycans. Cultured human AoSMC, AoEC and fibroblasts served as experimental model for all major resident cells of the arterial wall. Cellular deposition of both ECM proteins and GAGs were stimulated by physiological concentrations of fucoidan in AoSMC and DFB in a dose dependent manner. As compared to physiological levels of ascorbic acid there was less stimulation by fucoidan for ECM deposition of collagens, especially collagen type IV. ECM deposition of heparan sulfate was stimulated even by low doses of fucoidan comparably to the effect of ascorbic acid in DFB. However fucoidan stimulation was significantly higher, exceeding the ascorbate effects in AoSMC. Neither fucoidan nor ascorbic acid had any modulating effects on deposition of tested ECM proteins and GAGs by cultured AoEC.
Absence of combined or synergistic effects between fucoidan and ascorbate. We noticed no enhanced effects from a combination of fucoidan and ascorbic acid, compared to their individual effects. In fact, addition of ascorbate sometimes inhibited stimulating effects of fucoidan, as was observed in the case of ECM deposition of heparan sulfate by AoSMC.
The results support our hypothesis on the possible role of fucoidan as a substitution for vitamin C deficiency as all effects of both compounds were in the same directions and with comparable magnitude. However, the nature of immunochemical assays, applied for characterization of ECM components deposition, does allow only quantitative estimations in terms of total mass of an ECM component deposition by cells. It does not evaluate the quality or particular properties of deposited ECM compounds. For instance, ascorbic acid not only stimulates collagen synthesis and deposition by AoSMC and fibroblasts, but also regulates proper collagen filaments assembly as a cofactor of its cross-linking enzymes [11]. The nature of collagen filaments assembly under action of fucoidan remains unknown. Such characterizations lay beyond the scope of this study and shall be addressed in future research.
Recently, it has been demonstrated that ascorbic acid also participates in regulation of expression of different genes responsible for production and deposition of different ECM components, both proteins and GAGs [17]. It is also a subject for future research to determine exact pathways of fucoidan – dependent stimulation of ECM components deposition, such as whether it affects processes of protein or glycosaminoglycan synthesis, secretion or activation of corresponding genes.
Differences in deposition of collagens and GAGs by different cell types. Interesting results have been brought a direct comparison of ECM components deposition by different arterial wall resident cell types under stimulation by the two compounds tested. In unstimulated cells a production of collagen type I was very low for all three cell types tested. And it was dramatically stimulated in very similar patterns by ascorbate or fucoidan in AoSMC and DFB. Both stimuli failed to affect collagen type I production in AoEC. In contrast, unstimulated AoEC produced dramatically high levels of collagen type IV as compared to other cell types, and addition of ascorbate or fucoidan did not alter these levels. Treatment with physiological concentrations of fucoidan or ascorbate caused significant increase in collagen type IV deposition by AoSMC and DFB, but resulting levels were still significantly lower than in AoEC.
Differences in heparan sulfate deposition in different cell types were more noticeable. Thus, in the absence of external stimuli, DFB produced significant levels of heparan sulfate as compared to low levels both in AoSMC and AoEC. Both stimuli caused further increase in DFB. Only fucoidan supplementation was able to bring levels of heparan sulfate deposition in AoSMC close to those observed in DFB.
Hyaluronic acid deposition levels were similar in all cell types cultured in plain growth media. Only AoSMC and DFB responded to tested stimulations. Response to ascorbate was significantly higher in DFB than in AoSMC, whereas fucoidan was more effective in AoSMC.
Interestingly enough, deposition of none of the tested ECM components were modulated in cultured AoEC.
Differences in deposition of collagens between cultured arterial wall resident cells in general reflect the differences in composition between basement membrane and inner layers of the arterial wall. Thus, in normal healthy basement membrane produced and deposited by AoEC, collagen type IV is more predominant than collagen type I. In contrast, in healthy inner layers of the arterial wall, produced and deposited by AoSMC, a predominant collagen species is type I collagen [5].
Evolutional meanings of these findings in connection with the Rath-Pauling hypothesis. Our results demonstrated that seaweed polysaccharide fucoidan can stimulate production and deposition of ECM components by different types of arterial wall resident cells independently of the effects of ascorbic acid. These findings make an important addition to the Rath-Pauling theory on the development of human cardiovascular disease as a result of chronic vitamin C deficiency. Apparently, during human evolution nature invented several ways of supporting human life during harsh periods when the sources of essential nutrients and vitamins became scarce. In addition to internal body factor lipoprotein(a), which functions as a temporary glue material to support structural mechanical properties of the weakening arterial wall, there may be other factors offering temporary support [12]. Notably, here we provide support for such a role to be played by seaweed-derived fucoidan, a natural compound available from sea and ocean waters as a source of nutrition for ancient man, which could support vascular wall health by assuring ECM components production in the absence of vitamin C.
As mentioned above the quality of such vitamin C substituting effects by fucoidan might be limited to merely increasing a bulk production of ECM components as opposed to the complete ascorbate effects, which include both an increase in bulk production of ECM collagens and their proper structural characteristics. As in the case of lipoprotein(a), when its beneficial vitamin C substituting actions for arterial wall stability are compromised by aggravating the development of cardiovascular disease under conditions of a life long subclinical ascorbate deficiency [18], the beneficial effects of fucoidan could be compromised by inferior quality of deposited ECM components and offer only a temporary solution. This is still an open question for further research.
Nevertheless, the findings described here mean that there could be more than one evolutional mechanism, both of internal and external origin, which could provide a temporary substitutive role for vitamin C under conditions of its acute or chronic deficiency. In addition, such substitutive factors could cooperate in their beneficial action possibly forming a synergistic relationship since different molecular mechanisms of action are involved.
In conclusion, the results support our initial hypothesis on a capacity of seaweed sulfated polysaccharide fucoidan to possess vitamin C-like activity on ECM components production and deposition by arterial wall resident cells.
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015; 385:117-71.
- Lusis A.J. Atherosclerosis. Nature. 2000; 407: 233–241.
- Ross R. Cell biology of atherosclerosis. Annu. Rev. Physiol. 1995; 57: 791-804.
- Wight TN. The vascular extracellular matrix. In: Fuster V, Ross R, Topol EJ, ed. Atherosclerosis and coronary artery disease. Philadelphia: Lippincott-Raven; 1996: 421-440.
- Shekhonin BV, Domogatsky SP, Idelson GL, Koteliansky VE, Rukosuev VS. Relative distribution of fibronectin and type I, III, IV, V collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis. 1987; 67: 9-16.
- Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005; 6(7): 530-41.
- Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 2014; 10(4): 1558-70.
- Mills EJ, Wu P, Chong G, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170, 255 patients from 76 randomized trials. QJM. 2011; 104: 109–124.
- Maruthur NM, Wang N, Appel LJ. Lifestyle interventions to reduce coronary heart disease risk: results from the PREMIER trial. Circulation. 2009; 119: 2026-31.
- Tuso P, Stoll SR, Li WW. A plant-based diet, atherogenesis, and coronary artery disease prevention. Perm J. 2015; 19(1): 62-7.
- Levine M, Rumsey SC, Wang Y, Park JB, Daruwala R. Vitamin C. In: Stipanuk MH, (ed). Biochemical and Physiological Aspects of Human Nutrition. Philadelphia: WB Saunders; 2000: 541-567.
- Rath M, Pauling L. Unified Theory of Human Cardiovascular Disease Leading the Way to the Abolition of This Disease as a Cause for Human Mortality. J Orthomol Med. 1992; 7: 5-15.
- Cha J, Niedzwiecki A, Rath M. Hypoascorbemia induces atherosclerosis and vascular deposition of lipoprotein(a) in transgenic mice. Am J Cardiovasc Dis 2015; 5: 53-62
- Hosomi R, Yoshida M, Fukunaga K. Seafood consumption and components for health. Glob J Health Sci. 2012; 4(3): 72-86.
- Fitton JH, Stringer DN, Karpiniec SS. Therapies from Fucoidan: An Update. Mar Drugs. 2015; 13(9): 5920-46.
- Ivanov V, Ivanova S, Kalinovsky T, Niedzwiecki A, Rath M. Plant-derived micronutrients suppress monocyte adhesion to cultured human aortic endothelial cell layer by modulating its extracellular matrix composition. J Cardiovasc Pharmacol. 2008; 52(1): 55-65.
- Hitomi K, Tsukagoshi N. Role of ascorbic acid in modulation of gene expression. Subcell Biochem. 1996; 25: 41-56.
- Malaguarnera M, Vacante M, Russo C, et al. Lipoprotein(a) in cardiovascular diseases. Biomed Res Int. 2013; 650989.
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015; 385:117-71.
- Lusis A.J. Atherosclerosis. Nature. 2000; 407: 233–241.
- Ross R. Cell biology of atherosclerosis. Annu. Rev. Physiol. 1995; 57: 791-804.
- Wight TN. The vascular extracellular matrix. In: Fuster V, Ross R, Topol EJ, ed. Atherosclerosis and coronary artery disease. Philadelphia: Lippincott-Raven; 1996: 421-440.
- Shekhonin BV, Domogatsky SP, Idelson GL, Koteliansky VE, Rukosuev VS. Relative distribution of fibronectin and type I, III, IV, V collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis. 1987; 67: 9-16.
- Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005; 6(7): 530-41.
- Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 2014; 10(4): 1558-70.
- Mills EJ, Wu P, Chong G, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170, 255 patients from 76 randomized trials. QJM. 2011; 104: 109–124.
- Maruthur NM, Wang N, Appel LJ. Lifestyle interventions to reduce coronary heart disease risk: results from the PREMIER trial. Circulation. 2009; 119: 2026-31.
- Tuso P, Stoll SR, Li WW. A plant-based diet, atherogenesis, and coronary artery disease prevention. Perm J. 2015; 19(1): 62-7.
- Levine M, Rumsey SC, Wang Y, Park JB, Daruwala R. Vitamin C. In: Stipanuk MH, (ed). Biochemical and Physiological Aspects of Human Nutrition. Philadelphia: WB Saunders; 2000: 541-567.
- Rath M, Pauling L. Unified Theory of Human Cardiovascular Disease Leading the Way to the Abolition of This Disease as a Cause for Human Mortality. J Orthomol Med. 1992; 7: 5-15.
- Cha J, Niedzwiecki A, Rath M. Hypoascorbemia induces atherosclerosis and vascular deposition of lipoprotein(a) in transgenic mice. Am J Cardiovasc Dis 2015; 5: 53-62
- Hosomi R, Yoshida M, Fukunaga K. Seafood consumption and components for health. Glob J Health Sci. 2012; 4(3): 72-86.
- Fitton JH, Stringer DN, Karpiniec SS. Therapies from Fucoidan: An Update. Mar Drugs. 2015; 13(9): 5920-46.
- Ivanov V, Ivanova S, Kalinovsky T, Niedzwiecki A, Rath M. Plant-derived micronutrients suppress monocyte adhesion to cultured human aortic endothelial cell layer by modulating its extracellular matrix composition. J Cardiovasc Pharmacol. 2008; 52(1): 55-65.
- Hitomi K, Tsukagoshi N. Role of ascorbic acid in modulation of gene expression. Subcell Biochem. 1996; 25: 41-56.
- Malaguarnera M, Vacante M, Russo C, et al. Lipoprotein(a) in cardiovascular diseases. Biomed Res Int. 2013; 650989.

