Hair growth stimulating effect of a nutrient mixture in athymic nude mice
Alopecia, a common, distressing occurrence in humans also occurs in rodents.
Our main objective in this study was to examine the effects of a mixture of nutrients containing ascorbic acid, lysine, proline and green tea extract on hair growth in nude mice, since they are genetically predisposed to pattern balding.
Prior to testing the nutrients on nude mice, we studied the effect of NM by diet and topical application on hair growth in shaved dorsal region of Swiss mice. Following one week of isolation, dorsal hairs were shaved with an electric shaver (3 x 2 cm) and removed from shaved area with PBS.
After 24h the animals (n = 15) were divided into three groups of five each and subjected to the following treatment.
- Group 1 mice were fed regular Purina mouse chow diet for four weeks and 200µl of olive oil was applied to the shaved area three times a week for four weeks.
- Group 2 mice were fed Purina mouse chow diet for four weeks and 250µg NM in 200µl of olive oil was applied to the shaved area three times a week for four weeks.
- Group 3 mice were fed the regular diet supplemented with 0.5% NM (w/w) for four weeks and 200µl of olive oil was applied to the shaved area three times a week for four weeks.
Hair growth and weight were monitored and photographed for four weeks for each group. Hair growth in Groups 1 and 3 were comparable. However, in group 2, in which NM applied directly, the hair growth was significantly increased and the whole shaved area was covered with new hairs. From these observational results, which suggested that direct application of NM was more beneficial than NM in diet or olive oil application, we proceeded to the study of NM in athymic nude mice. Following one week of isolation, the mice were divided into two groups of three mice each and fed the regular Purina mouse diet for three weeks and subjected to the following treatments. Group 1 mice received applications of 200µl of olive oil to the dorsal area three times a week for three weeks. Group 2 mice received applications of 250µg NM in 200µl of olive oil to the dorsal area three times a week for three weeks. Hair growth and weight were monitored and photographed for four weeks for each group.
After three weeks of NM in olive oil application there was significant growth of hair in group 2 nude mice, suggesting that even in athymic mice NM stimulated hair growth.
These results suggest that the NM may be useful in treatment of some forms of alopecia in humans.
Keywords
Athymic nude mice;
hair growth;
nutrient mixture;
Correspondence to
Dr. Aleksandra Niedzwiecki
Dr. Rath Research Institute
1260 Memorex Drive
Santa Clara, CA 95050, USA
Email: author@jcmnh.org
Introduction
Hair loss, a common occurrence in humans, also occurs in rodents. In rodents, hair loss is reported to occur spontaneously due to overgrooming behavior, stress and skin inflammation1,2,3,4. In previous studies, a nutrient mixture containing vitamin C, lysine, proline and green tea extract has demonstrated anti-inflammatory5 and skin cancer preventative effects6. Estandiari and Kelly reported significant hair regrowth in female Balb/black mice that had experienced spontaneous hair loss by administering green tea-derived polyphenolic extract in their water7. Vegesna et al. demonstrated hair growth in nude mice treated with vitamin D3 analogs8.
Our main objective in this study was to examine the effects of a mixture of nutrients containing ascorbic acid, lysine, proline and green tea extract on hair growth in nude mice, since they are genetically predisposed to pattern balding. Prior to testing the nutrients on nude mice, we studied the effect of NM by diet and topical application on hair growth in shaved dorsal region of Swiss mice to determine which NM delivery system was more effective.
Materials and Methods
The nutrient mixture (NM), prepared by VitaTech (Hayward, CA) was composed of the following ingredients in the relative amounts indicated: Vitamin C (as ascorbic acid and as Mg, Ca, and palmitate ascorbate) 700 mg; L-lysine 1000 mg; L-proline 750 mg; L-arginine 500 mg; N-acetyl cysteine 200 mg; standardized green tea extract (80% polyphenol) 1000 mg; selenium 30 μg; copper 2 mg; manganese 1 mg. All other reagents used were of high quality and were obtained from Sigma, unless otherwise indicated.
Animals. Fifteen male Swiss mice and six male nude mice free of murine virus, bacteria and parasites, approximately six to seven weeks of age on arrival, were purchased from Simonsen Laboratory (Gilroy, CA, USA). The mice were housed in microisolator plastic cages in a room with controlled temperature, airflow, pathogen free and humidity under a 12h light/12h dark cycle. The care and handling of the animals were performed according to a protocol approved by an internal institutional Animal Safety Review Committee and followed the humane and customary care of experimental animals.
Experimental design
Effect of NM on hair growth in male Swiss mice
Following one week of isolation, dorsal hairs were shaved with an electric shaver (3 x 2 cm) without causing injury to the skin. Hairs were removed from shaved area with PBS. After 24h the animals were divided into three groups of five each and subjected to the following treatment. Group 1 mice were fed regular Purina mouse chow diet for four weeks, 200µl of olive oil was applied to the shaved area three times a week for four weeks. Group 2 mice were fed Purina mouse chow diet for four weeks and 250µg NM in 200µl of olive oil was applied to the shaved area three times a week for four weeks. Group 3 mice were fed the regular diet supplemented with 0.5% NM (w/w) for four weeks and 200µl of olive oil was applied to the shaved area three times a week for four weeks. Hair growth and weight were monitored and photographed for four weeks for each group.
Effect of NM on hair growth in athymic nude mice
After one week of isolation, the mice were divided into two groups of three mice each and fed the regular Purina mouse diet for three weeks and subjected to the following treatments. Group 1 mice received application of 200µl of olive oil to the dorsal area three times a week for three weeks. Group 2 mice received application of 250µg NM in 200µl of olive oil to the dorsal area three times a week for three weeks. Hair growth was monitored and photographed for three weeks for both groups.
Results
Hair growth in Swiss mice. Hair growth in groups 1 and 3 were comparable. However, in group 2, in which NM was applied directly, the hair growth was significantly increased and the whole shaved area was covered with new hair. These observational results suggested that direct application of NM was more beneficial than NM in diet or olive oil application.
See Figures 1 – 3.
There were no significant differences in mean body weights of the three groups.
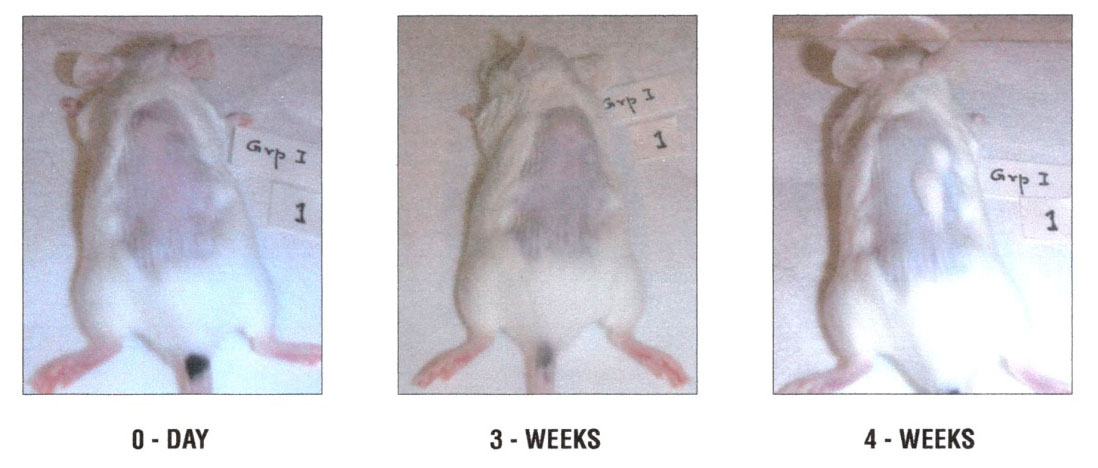
Figure 1. Representative control group male swiss mice treated with 200µl of olive oil: 1A – 0 days, 1B – 3 weeks, 1C – 4 weeks
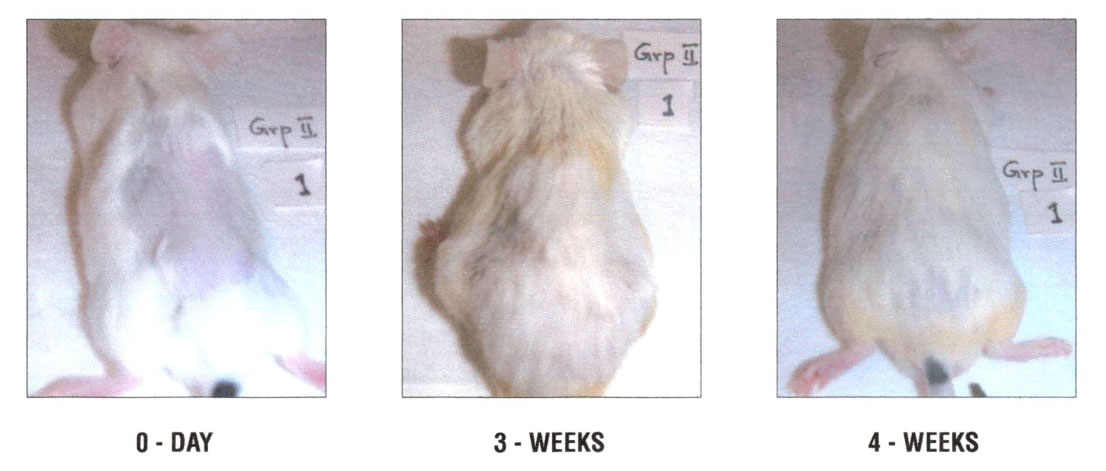
Figure 2 – Representative male swiss mice treated with application of NM 250µg in 200µl of olive oil: 2A – 0 days, 2B – 3 weeks, 2C – 4 weeks
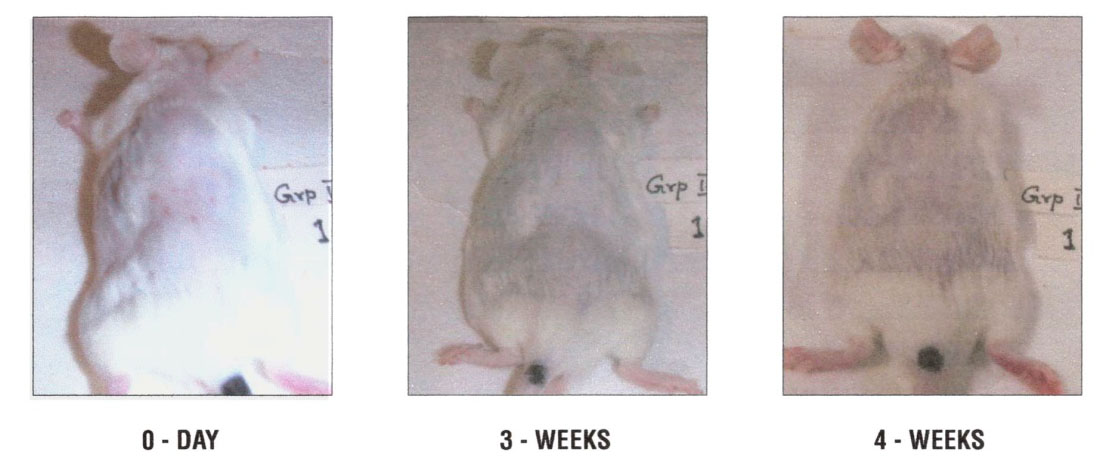
Figure 3 – Representative male swiss mice treated with dietary NM 0.5%: 3A – 0 days, 3B – 3 weeks, 3C – 4 weeks
Hair growth in athymic nude mice. After three weeks of NM in olive oil application there was significant growth of hair in the NM-treated nude mice, in contrast to no hair growth in the control group, suggesting that even in athymic mice NM stimulated hair growth. See Figure 4.
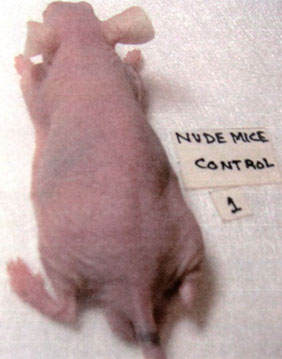
4A. Control group
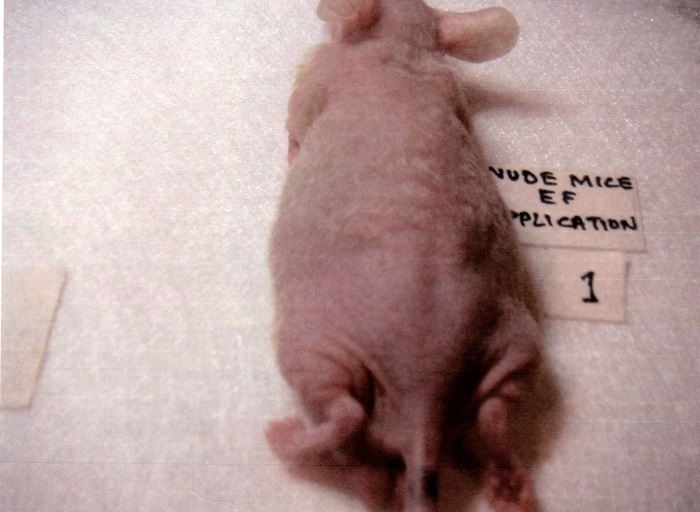
4B. NM-treated group
Figure 4 – Representative control and NM-treated male nude mice: 4A – Control, 4B – NM 250µg in 200µl of olive oil
Discussion
The nude mouse (nu / nu), a strain of mice that spontaneously arose in an albino strain, has been found to be a pleiotropic mutation, that phenotypically expresses a disturbed development of hair follicles and dysgenesis of the thymus, which is arrested in an early stage9. Though nude mice have been used extensively due to their impaired T-cell function, few investigations have been published on the cutaneous abnormalities in nude mice10,11. Once Foxn1 was recognized as the nude gene, nude mouse skin research significantly increased12,13. Many investigations indicate that Foxn1 is possibly involved in keratinocyte differentiation. Meier et al reported that Foxn1 is an important regulator of an acidic hair keratin gene, suggesting that nude represents the first inherited skin disorder that is caused by a loss rather than aberrant expression of a keratin gene14. Nude mice lack vibrissae at birth, in contrast to heterozygote wildtype mice, but have well developed hair bulbs of vibrissae 24h after birth. Instead of penetrating the dorsal skin, hair shafts bend and coil when entering the hair canal10,15. During anagen phase, nude mice develop visible sparse and fine hair, which are lost again during the catagen phase10.
Though the biological role of Foxn1 is not fully understood, natural products, such as chrysanthemum zawadski and other medicinal plant extracts have shown increased number of hair follicles and follicular keratinocyte proliferation in nude mice16. Vegesna et al. demonstrated hair growth in nude mice treated with vitamin D3 analogs8. Cyclosporin A was also found to stimulate hair growth in nude mice17.
In our studies, not only did application of the nutrient mixture stimulate hair growth in shaved dorsal areas of normal Swiss mice, but also stimulated growth of hair in nude mice. Though further studies are warranted to elucidate the mechanisms involved, our results suggest that the NM may be useful in treatment of some forms of alopecia in humans.
Acknowledgments
The research study was funded by Dr. Rath Health Foundation (Santa Clara, CA, USA), a non-profit organization.
References
- Litterst CL. Mechanically self-induced muzzle alopecia in mice. Lab Anim Sci. 1974; 24(5): 806–809.
- Thornburg LP, Stowe HD,mPick JR. The pathogenesis of the alopecia due to hair-chewing in mice. Lab Anim Sci. 1973; 23(6): 843–850.
- Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a ‘brain-hair follicle axis (BHA)’: inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J. 2001; 15(13): 2536–2538.
- Arck PC, Handjiski B, Peters EMJ, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003; 162(3): 803–814.
- Ivanov V, Cha J, Ivanova S, et al. Essential nutrients suppress inflammation by modulating key inflammatory gene expression. Int J Mol Med. 2008; 22(6): 731-741.
- Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M. Anticancer effects of a micronutrient mixture on melanoma: modulation of metastasis and other critical parameters. In: Breakthroughs in Melanoma Research, Tanaka Y (ed.), InTech Publisher; 2011: 559-574.
- Esfandiari A, Kelly AP. The effects of tea polyphenolic compounds on hair loss among rodents. J Natl Med Assoc. 2005; 97(8): 1165-1169.
- Vegesna V, O’Kelly J, Uskokovic M, et al. Vitamin D3 analogs stimulate hair growth in nude mice. Endocrinology. 2002; 143(11): 4389-96.
- Nehls M, Kyewski B, Messerle M, et al. Two genetically separable steps in the differentiation of thymic epithelium. Science. 1996; 272: 886-889.
- Flanagan SP. “Nude,” a new hairless gene with pleiotropic effects in the mouse. Genet Res Comb. 1966; 8: 295-309.
- Köpf-Maier P, Mboneko VF, Merker HJ. Nude mice are not hairless. A morphological study. Acta Anat (Basel). 1990; 139(2): 178-190.
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000; 14: 142-146.
- Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994; 372: 103–107.
- Meier N, Dear TN, Boehm T. Whn and mHa3 are components of the genetic hierarchy controlling hair follicle differentiation. Mech Dev. 1999; 89: 215–221.
- Rigdon RH, Packchanian AA. Histologic study of the skin of congenitally athymic “nude” mice. Texas Rep Biol Med. 1974; 32: 711-723.
- Belgum S, Gu LJ, Lee MR, et al. In vivo hair growth-stimulating effect of medicinal plant extract on BALB/c nude mice. Pharm Biol. 2015; 53(8): 1098-1103.
- Sawada M, Terada N, Taniguchi H, Tateishi R, Mori Y. Cyclosporin A stimulates hair growth in nude mice. Lab Invest. 1987; 56(6): 684-6.
- Litterst CL. Mechanically self-induced muzzle alopecia in mice. Lab Anim Sci. 1974; 24(5): 806–809.
- Thornburg LP, Stowe HD,mPick JR. The pathogenesis of the alopecia due to hair-chewing in mice. Lab Anim Sci. 1973; 23(6): 843–850.
- Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a ‘brain-hair follicle axis (BHA)’: inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J. 2001; 15(13): 2536–2538.
- Arck PC, Handjiski B, Peters EMJ, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003; 162(3): 803–814.
- Ivanov V, Cha J, Ivanova S, et al. Essential nutrients suppress inflammation by modulating key inflammatory gene expression. Int J Mol Med. 2008; 22(6): 731-741.
- Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M. Anticancer effects of a micronutrient mixture on melanoma: modulation of metastasis and other critical parameters. In: Breakthroughs in Melanoma Research, Tanaka Y (ed.), InTech Publisher; 2011: 559-574.
- Esfandiari A, Kelly AP. The effects of tea polyphenolic compounds on hair loss among rodents. J Natl Med Assoc. 2005; 97(8): 1165-1169.
- Vegesna V, O’Kelly J, Uskokovic M, et al. Vitamin D3 analogs stimulate hair growth in nude mice. Endocrinology. 2002; 143(11): 4389-96.
- Nehls M, Kyewski B, Messerle M, et al. Two genetically separable steps in the differentiation of thymic epithelium. Science. 1996; 272: 886-889.
- Flanagan SP. “Nude,” a new hairless gene with pleiotropic effects in the mouse. Genet Res Comb. 1966; 8: 295-309.
- Köpf-Maier P, Mboneko VF, Merker HJ. Nude mice are not hairless. A morphological study. Acta Anat (Basel). 1990; 139(2): 178-190.
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000; 14: 142-146.
- Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994; 372: 103–107.
- Meier N, Dear TN, Boehm T. Whn and mHa3 are components of the genetic hierarchy controlling hair follicle differentiation. Mech Dev. 1999; 89: 215–221.
- Rigdon RH, Packchanian AA. Histologic study of the skin of congenitally athymic “nude” mice. Texas Rep Biol Med. 1974; 32: 711-723.
- Belgum S, Gu LJ, Lee MR, et al. In vivo hair growth-stimulating effect of medicinal plant extract on BALB/c nude mice. Pharm Biol. 2015; 53(8): 1098-1103.
- Sawada M, Terada N, Taniguchi H, Tateishi R, Mori Y. Cyclosporin A stimulates hair growth in nude mice. Lab Invest. 1987; 56(6): 684-6.

