Comparison of the antioxidant efficacy and cellular protection by several categories of nutritional supplements on the market
Oxidative stress is a common source of cellular damage that is implicated in many diseases. Many people use nutritional supplements to maintain and improve their health. However, there is little information on how, or even if, popular dietary supplements improve cellular health by protecting the body from oxidative stress. Our study tests popular dietary supplements from the European and US markets in a uniform, standardized manner. This allows us to better understand how the differences in supplement compositions and/or ingredient doses may affect their efficacy at cellular level. The results show large differences in cellular efficacy of supplements even within the same category. Consistently, products containing ingredients chosen on the basis of their synergy confer greater protection from oxidative stress.
Keywords
oxidative stress;
antioxidants;
cultured cells;
dietary supplements;
Correspondence to
Dr. Aleksandra Niedzwiecki
Dr. Rath Research Institute
1260 Memorex Drive
Santa Clara, CA 95050, USA
Email: author@jcmnh.org
Introduction
The cells building our body face risk of oxidative damage from external agents and free radicals generated during normal cellular metabolism or resulting from different pathological processes. Therefore, they are equipped with natural mechanisms to scavenge free radicals and if needed, repair the damage to cellular organelles and structures as a way of maintaining tissue homeostasis. However, excessive production of reactive oxygen species and the body’s failure to rapidly counteract their deleterious effects on cells and tissues results in oxidative stress leading to accelerated aging and various pathologies. In addition to internal cellular anti-oxidant mechanisms, specific antioxidant vitamins and other nutrients present in some foods help in counteracting the negative effects of oxidative stress in our body1,2. As such, dietary intake of antioxidants-rich foods has shown to be associated with many health benefits including prevention of pathological conditions associated with high oxidative stress .i.e. cancer3, arthritis, Crohn disease, Alzheimer’s disease and many others4,5,6.
Based on accumulated scientific and epidemiological evidence supporting beneficial effects of antioxidants many people take nutritional supplements as a way of supplementing nutrition poor diets, correcting specific nutrient deficiencies or addressing higher demands for micronutrients based on genetic predisposition, environmental and life style challenges. According to Centers for Disease Control and Prevention the use of dietary supplements in the United States is increasing with 40 percent of the population 2 months of age and older taking a least one daily supplement with some taking multiple supplements a day7. Popularity of supplements use in Europe vary. For example it is common in Germany and Denmark (43% and 59% of the adult population respectively) but is less so in Ireland and Spain (23% and 9% respectively). Women use supplements more than men8,9,10,11,12.
There are numerous nutritional supplements on the market containing individual antioxidant nutrients, such as vitamin C, vitamin E, EGCG from Green tea, Resveratrol and many other compounds13,14. In addition, many manufacturers offer multi-nutrient supplements containing wide spectrum of vitamins, many of which combined with minerals, plant extracts and other nutritional components and claiming their efficacy in protection against free radicals generated damage. However, the basis for selection of specific ingredients, the use of specific doses and combinations of components and sources of raw ingredients in these formulas is not clearly explained. Different nutrients which are claimed to possess antioxidant capacity are often added to other compounds with no regard to how, or if, the different nutrients within a supplement interact in a synergistic, additive or antagonistic ways or do not interact at all. Moreover, rarely or not at all, the manufacturers of these products present scientific proofs documenting the efficacy of their formulas at the biochemical and cellular levels. It is generally accepted that the quality of ingredients (raw materials) used in nutritional supplements is important in assuring efficacy and safety of the products, as many synthetic ingredients have been shown to be harmful or not effective15. While, some manufacturers stress the use of natural sources of ingredients in promoting their products they do not provide proofs whether it results in better product efficacy at the cellular level. As such, in the majority of supplements, the structure-function claims of a product are backed up either by general statements taken from the literature or purely on marketing slogans.
The aim of this study was to compare the efficacy of different nutritional supplements marketed in EU and USA in protecting cells from oxidative stress. This evaluation included the measurements of total antioxidant potential of various formulations expressed as their ability to quench the long-lived reactive oxygen species in comparison to Trolox, a water-soluble vitamin E analog. In addition, we evaluated cell-protective effects of these supplements against oxidative stress induced by in vitro exposure of human aortic smooth muscle and human kidney cells to hydrogen peroxide.
Basis for selecting products. We selected popular and high-end nutritional supplements sold in European nations and USA. We compared products developed at our Institute against those manufactured by other companies. In total, we tested six of our products and eight manufactured by other manufacturers. For ethical reasons, we anonymized all the products. Since the formulations are all different, we categorized them as follows:
- Set A- Vitamin C based products: These products state vitamin C (in different forms) as the primary ingredient and may or may not contain bioflavonoids. Four products tested
- Set B- Multi-nutrient products: These multi-nutrient products contain combinations of most popular vitamins, minerals and trace elements as well as other active compounds. Nine products tested.
- Set C- Bioflavonoids: One product containing a mixture of bioflavonoids without vitamins and other micronutrients was tested in this category.
Since all these products differed not only in nutrient composition, but also in nutrient doses, we compared their efficacy based on a recommended daily intake by the manufacturer of each product assuming that this reflects effective dose of a formulation. Three sets of products were further divided into two subsets each based on whether the manufacturers claimed that the ingredients were based on synergy or the nutrient combination was random (or not specified by the manufacturer). Based on this, we created the column “Synergy Claimed” in Table 1.
Table 1: Ingredient composition of tested supplements. The numbers of different vitamins, minerals, active compounds, amino acids or plant extracts in each product are listed in parenthesis. Different forms of a particular vitamin, for instance, mineral forms of Ascorbate and fat soluble form of Ascorbate, were counted as two distinct vitamins.
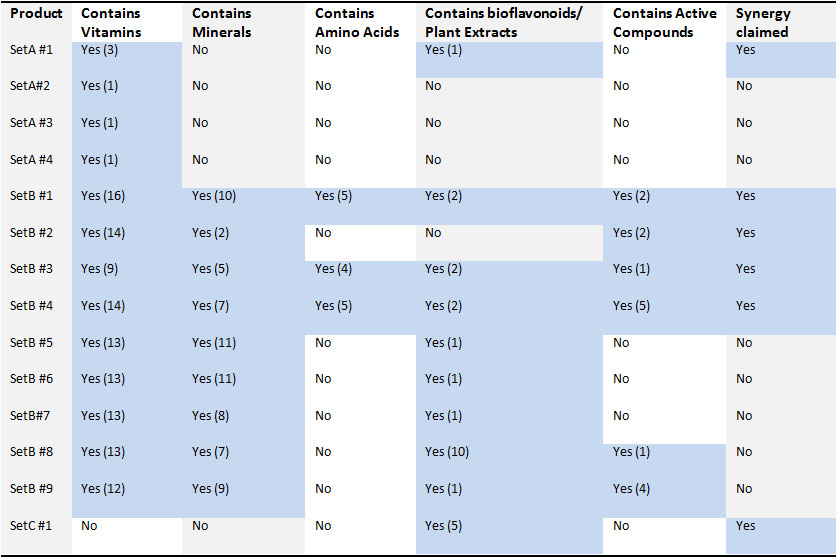
The ingredient composition of the products is provided in more detail in the table below. Five kinds of ingredients are found in nutritional supplements: vitamins, minerals, amino acids, plant extracts and active compounds. Active compounds include Coenzyme Q10, Phosphatidylserine, or Inositol that are essential for biochemical processes in the body but are not classified as vitamins, minerals or bioflavonoids.
Materials
Cell lines. Human Embryonic Kidney Cells (HEK-293) were obtained from ATCC (American Type Culture Collection, Rockville, MD, USA) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) and supplemented with 10% FBS, 100U/ml penicillin and 100U/ml streptomycin. Normal Human Primary Aortic Smooth Muscle Cells (AoSMC) were obtained from ATCC and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) and supplemented with 5% FBS, 100U/ml penicillin and 100U/ml streptomycin.
Chemicals. Hydrogen Peroxide was obtained from Sigma-Aldrich St. Louis, MO, USA. Cell viability kit CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) was obtained from Promega, Madison, WI, USA. Protein biomass was measured by In Vitro Toxicology Assay Kit, (Sulforhodamine B based) and was obtained from Sigma-Aldrich St. Louis, MO, USA. Trolox Equivalent Antioxidant Capacity (TEAC) was measured by Antioxidant Assay Kit obtained from Sigma-Aldrich St. Louis, MO, USA. Cat# CS0790. Hematoxylin and Eosin stains were obtained from EMD Millipore Corporation, Billerica, MA, USA. Images were taken on a Nikon Daiphot 300 microscope.
Preparation of Nutritional Supplements. All Nutritional Supplements were treated identically in accordance with the protocol recommended by Unites States Pharmacopeia16. Three recommended daily doses of each supplement were powdered (tablets were crushed using ceramic pestle and mortar; capsules were cut open and powder poured out), placed into glass container with 900 ml of 0.1N hydrochloric acid and incubated for 1 hour at 37°C in a shaking incubator set with rotation speed of 75 rpm. Resulting solutions were filter-sterilized using 0.2 micrometer pore size filters, aliquoted and kept frozen at -20°C until analyses. Amounts of samples taken for analysis are expressed as number of millionth parts of recommended daily dose of the respective supplement.
Methods
Cell viability assay. The assay was performed using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) supplied by Promega, Madison, WI, USA. This is a colorimetric assay for assessing cell metabolic activity. NAD(P)H-dependent cellular oxidoreductase enzymes in viable cells reduce the tetrazolium dye MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to formazan. The concentration of formazan produced is measured by optical density and it is directly proportional to the number of live cells in culture. Human Aortic Smooth Muscle (AoSM) Cells were seeded at 3×104 cells/ml in 96 well plates and grown in DMEM supplemented in 5% FBS for 5 days. Cells were pretreated with 0.25 millionth part daily doses of all prepared nutrition mixtures in a total reaction volume of 100 μl per mixture. After 24 hours of pretreatment, cells were washed with PBS and exposed to 1.5mM H2O2 dissolved in serum free DMEM for 30 minutes. After exposure cells were washed with PBS and incubated in DMEM supplemented with 1% BSA for 24 hours to allow for recovery. After the 24 hour recovery phase cells were washed with PBS and viability assay was performed as described in the accompanying protocol.
Protein Biomass Assay. Human Embryonic Kidney (HEK) cells were seeded at 3×104 cells/ml in 96 well plates and grown in DMEM supplemented in 10% FBS for 5 days. Cells were pretreated with 0.25 millionth part daily doses of all prepared nutrition mixtures in a total reaction volume of 100 μl per mixture. After 24 hours of pretreatment, cells were washed with PBS and exposed to 1 mM H2O2 dissolved in serum free DMEM for 30 minutes. After exposure cells were washed with PBS and incubated in DMEM supplemented with 1% BSA for 24 hours to allow for recovery. After the 24 hour recovery phase cells were washed with PBS and subjected to a 40 hour growth phase in DMEM supplemented in 10% FBS for 2 days. After the growth phase, the protein biomass assay was performed according to the accompanying protocol. Briefly, cell proteins were fixed, and stained with the dye. The incorporated dye was then liberated from the cells with a Tris base solution and quantified by optical density. An increase or decrease in the number of cells (total biomass) defines the amount of dye incorporated by the living cells in the culture as the dead cells are washed away. The optical density therefore determines total protein biomass.
Antioxidant capacity Assay. Assay was carried out as per manufacturer’s instructions (Sigma-Aldrich, Cat# CS0790). Product samples prepared as described above were used in assay at 75 millionth daily doses.
Morphology. Cells were grown in 48 well plates and subjected to the same conditions as in the cell viability and protein biomass assays. But instead of performing the assays, the cells were stained with Hematoxylin and Eosin dyes, observed under a light microscope and representative photos were taken.
Statistics. Results are presented as average of data obtained from triplicate or quadruplicate sets. The set number is specified in each figure. Error bars in each figure represent standard deviation.
Total antioxidant capacity (TEAC units). Among vitamin C based products, which means that vitamin C in a single or different chemical forms was a predominant ingredient, the greatest antioxidant capacity was detected with A#1 product. The rest of the vitamin C products in this Set was 3-6 times less potent. Among multi-nutrient formulas (Set B), the greatest antioxidant capacity was detected in products B#2, B#3 which had TEAC values comparable with A#1 (Fig.1). Interestingly, some of the multi-nutrient products did not have any recordable TEAC antioxidant capacity (B #5, 6, 7 and 9) despite containing antioxidant ingredients (i.e. vitamins) or other antioxidant compounds. This may be attributed either to too low doses of antioxidant ingredients, their low quality or their counteracting interactions with other compounds in the mixture. The product of Set C exhibited lower antioxidant capacity, and comparable to B6 which had high number of bioflavonoids/plant extract components in comparison to other B set products.
Results
Total antioxidant capacity (TEAC units). Among vitamin C based products, which means that vitamin C in a single or different chemical forms was a predominant ingredient, the greatest antioxidant capacity was detected with A#1 product. The rest of the vitamin C products in this Set was 3-6 times less potent. Among multi-nutrient formulas (Set B), the greatest antioxidant capacity was detected in products B#2, B#3 which had TEAC values comparable with A#1 (Fig.1). Interestingly, some of the multi-nutrient products did not have any recordable TEAC antioxidant capacity (B #5, 6, 7 and 9) despite containing antioxidant ingredients (i.e. vitamins) or other antioxidant compounds. This may be attributed either to too low doses of antioxidant ingredients, their low quality or their counteracting interactions with other compounds in the mixture. The product of Set C exhibited lower antioxidant capacity, and comparable to B6 which had high number of bioflavonoids/plant extract components in comparison to other B set products.
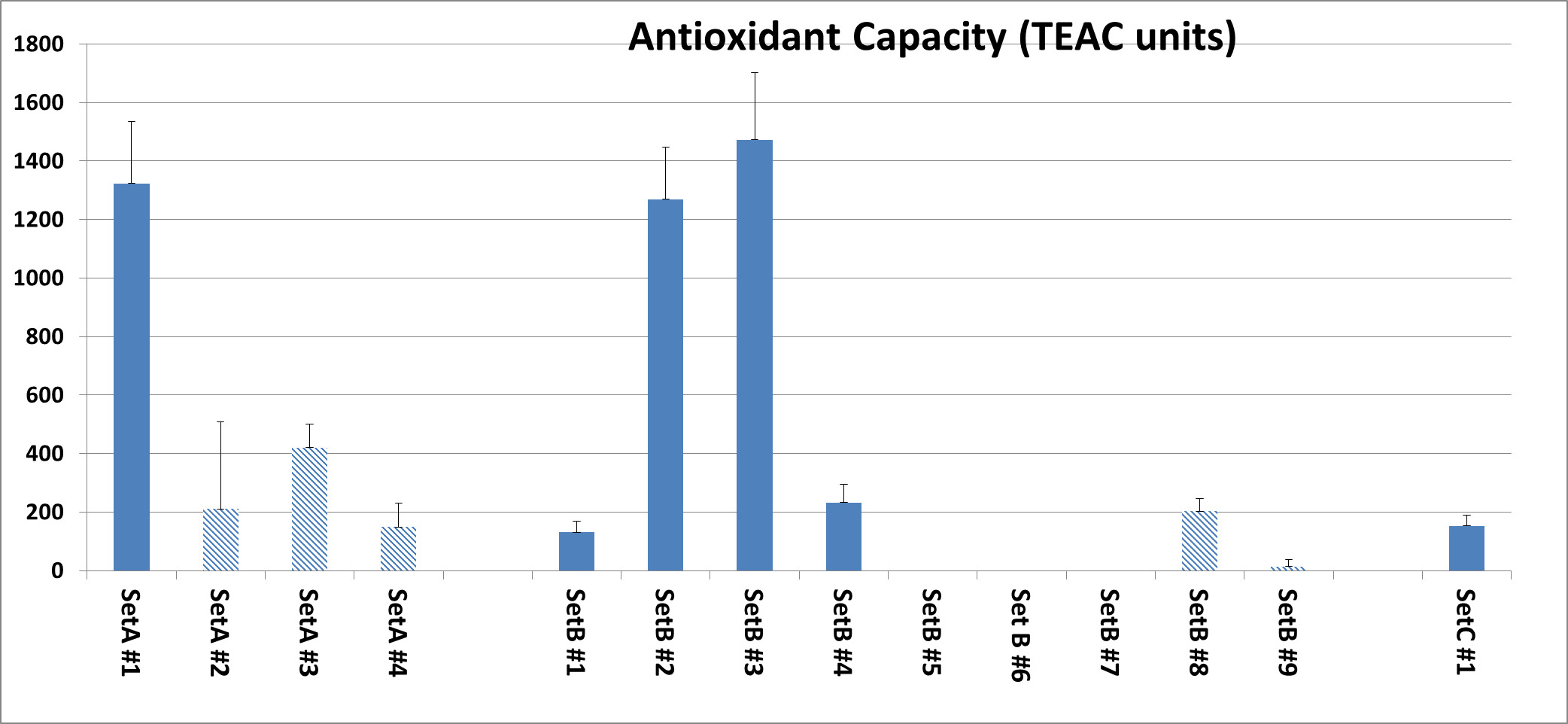
Fig. 1: Antioxidant capacity of tested supplements organized according to sets as described in Material and Methods. Results shown are an average of three separate experiments. All products were tested at 7 millionth part daily dose. Products manufactured by other companies are represented by shaded bars while our own formulations are represented by solid bars.
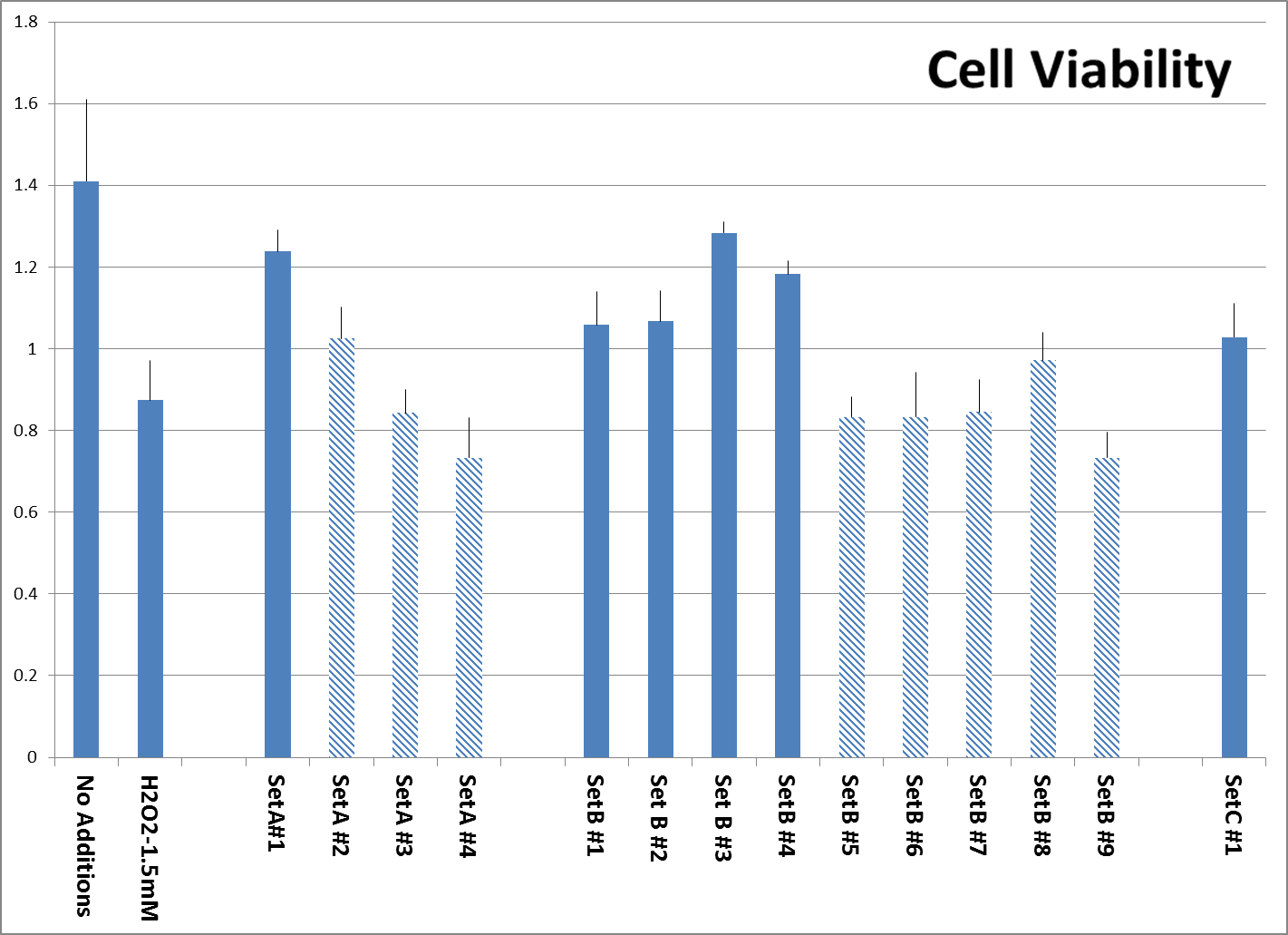
Fig. 2: Viability of additional surviving Human Aortic Smooth Muscle cells after pre-treatment with tested supplements, followed by H2O2 treatment as described, organized according to sets as described. Experiment was performed in triplicate. All products were tested at 0.25 millionth part of recommended daily dose. Viable cells reduce MTT reagent to a formazan product, which is estimated by optical density. Products manufactured by other companies are represented by shaded bars while both controls and our own formulations are represented by solid bars.
This assay however, does not tell us how the products may act on human cells exposed to oxidative stress. Therefore, in further experiments we tested the efficacy of these formulas in protecting cells against damage from H2O2 using cell culture systems.
Protection of Cell viability against H2O2-induced toxicity. The viability of Aortic Smooth Muscle cells exposed to H2O2 was tested as described in Material and methods. The concentration of H2O2 was selected to kill about 50% of the cells in a short exposure time because H2O2 is unstable and therefore longer treatments should be avoided. The results on Fig. 2 show that more cells survived the treatment with 1.5mM H2O2 in samples pretreated with supplements which contained bioflavonoids and claimed to contain ingredients based on nutrient synergy (A#1, and B #1,2,3,4) compared to products B# 5,6,7,8,9, which were not synergy based. Product C#1 provided cell protection equal to some Set B products.
Maintaining Cell Biomass after exposure to H2O2. Apart from cell viability, we assessed the protective effects of the supplements by measuring total protein biomass of surviving cells after their pre-treatment with tested supplements, followed by H2O2 exposure. This gives us an indication of how the structural stability of cells may be affected by nutrient supplementation. Fig. 3 shows that overall Set B and Set C products were more effective in preserving cell biomass against H2O2 induced damage than Set A products. Within the sets, products based on synergy preserved biomass better than those which did not make that claim.
Cell Morphology. Figure 4 is a visual representation of the effect of both H2O2 damage and the protective effects of selected supplements on Human Embryonic Kidney cells. Consistent with our other assays we see that supplements showing greater protective effects leave more cells behind.
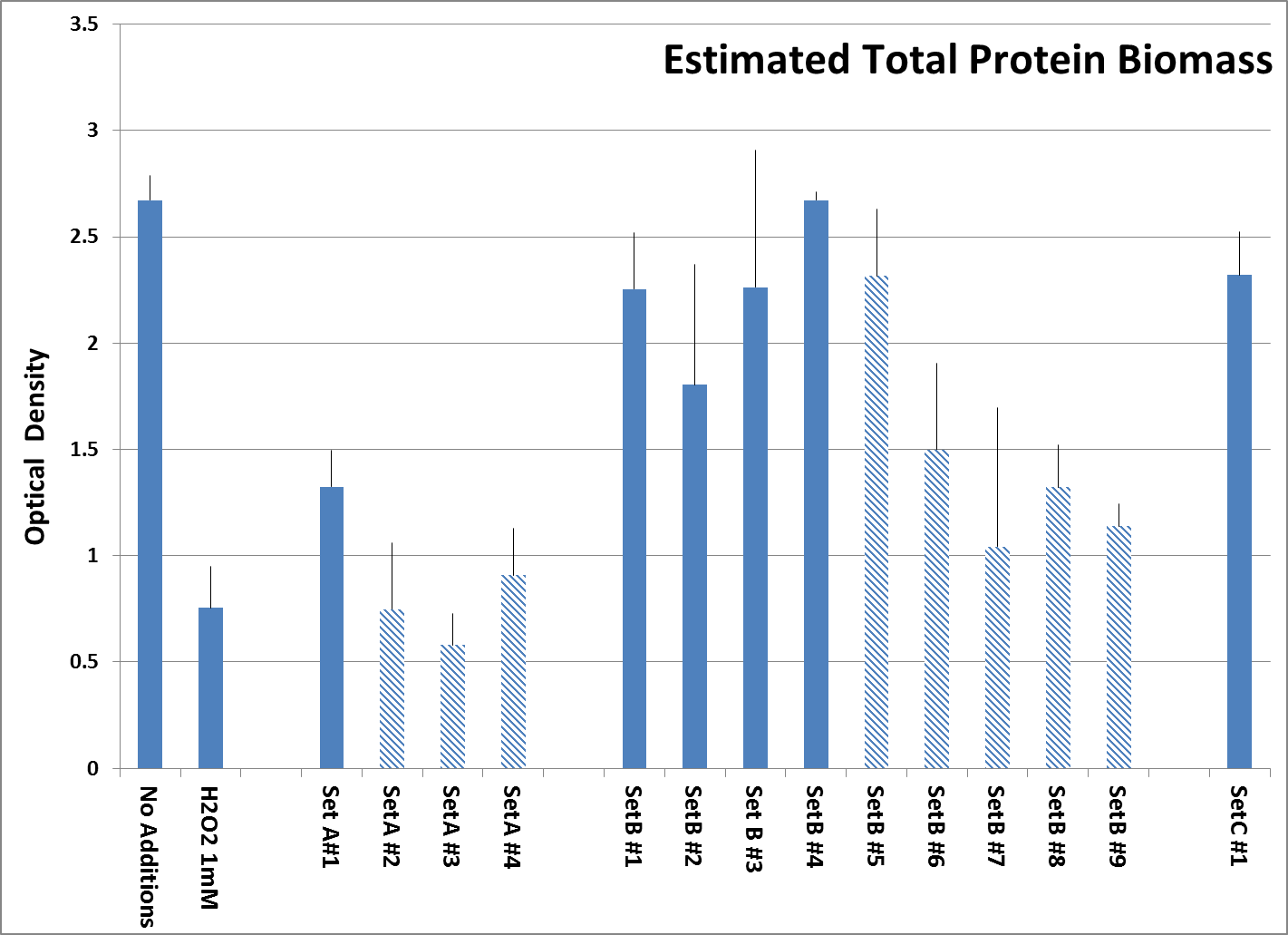
Fig. 3: Total Protein biomass of surviving Human Embryonic Kidney cells after pre-treatment with tested supplements, followed by H2O2 treatment as described, organized according to sets. Experiment was performed in quadruplicates. All products were tested at 0.25 millionth part daily dose. The bar labelled H2O2 1mM indicates the unprotected control which left only 28.2% of live protein biomass. Products manufactured by other companies are represented by shaded bars while both controls and our own formulations are represented by solid bars.
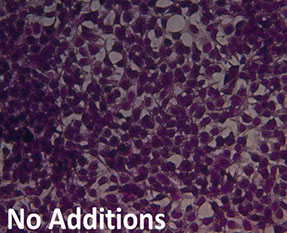

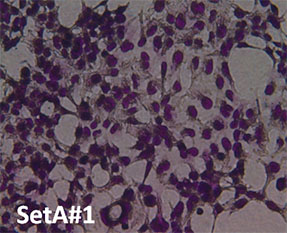
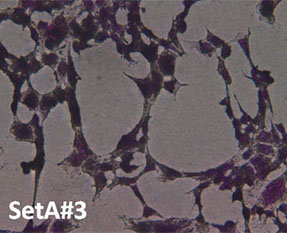
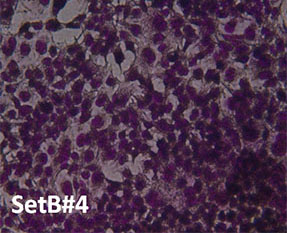
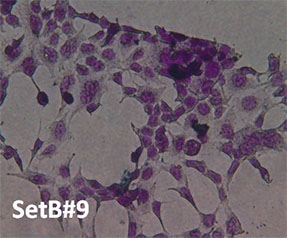
Fig. 4: Hemotoxylin and Eosin staining of surviving Human Embryonic Kidney cells after pre-treatment with tested supplements, followed by H2O2 treatment as described. All products were tested at 0.25 millionth part daily dose. Picture “No Additions” indicates the control cells treated only with cell growth media. Picture “No protection” shows the cells treated only with H2O2 1mM. Remaining pictures are labelled with the appropriate products applied to cells in the protection assays as described earlier.
Discussion
The results indicate that multivitamins and antioxidant containing products differ in their antioxidant capacity and ability to protect cells from oxidative stress. By testing popular supplement products offered on different markets, we attempted to find a rationale for the compositions of such supplements. Regardless of a method used in assessing effectiveness of supplements, in general, the formulations based on synergistic principles in nutrient composition confer greater cell protection from oxidative stress.
In order to better assess the efficacy of different categories of nutritional supplements containing antioxidant components we conducted three kinds of analyses; a cell free antioxidant capacity assay, a cell viability protection assay and a cell biomass protection assay, all of which allowed us to evaluate different aspects of their antioxidant potential. In this aspect TEAC (Trolox Equivalent Antioxidant Capacity) is a biochemical assay, which has been proposed as one of three assays considered for standardization of antioxidants. While TEAC provides indication of radical scavenging efficiency, it may not translate into antioxidant protection at the cellular level. This is because a biochemical assay does not take into account the damage and repair caused by oxidative stress on cellular proteins and other components. The repair and re-synthesis of cellular components damaged by oxidative stress is an important aspect of cell protection. Therefore we performed cell-based viability assays and biomass evaluation in addition to direct antioxidant capacity assay.
Cell viability and total biomass are both indirect methods of estimating cell protection. Cell viability tells us about how cell functioning is damaged by oxidative stress and protected by nutritional supplements, whereas biomass shows us the effects of oxidative stress on tissue integrity.
Despite variations in composition, we see that products containing a combination of vitamins, minerals, amino acids, active compounds and bioflavonoids are more consistently effective at combating oxidative stress than those lacking either of them. Regardless the basic composition of the products, we see that within each set, products whose ingredients were chosen based on synergistic principles outperform similar products of the same set. This explains the differences within the Set B and Set A products. Better performing nutritional supplement formulas in Set B contain vitamins, minerals, plant extracts, active compounds and amino acids while those that did not show effective protection lack at least some of those ingredients, in particular amino acids (Table 1). Interestingly, C#1 and B #1 through B #4 formulas showed similar, good efficacy in protecting cells against H2O2 damage both in terms of cell viability and biomass. This would suggest that bioflavonoids are important components of antioxidant synergy. Interestingly, non-synergy based products containing bioflavonoids and even active compounds did not show such high cell protecting potential. Differences in the quality of ingredients of the supplement and the amounts in a daily recommended dose may also explain the differences in efficacy within each set.
The results prove that not all micronutrient combinations are created equal. We hope our efforts at uniform testing of common nutritional supplements will encourage the manufacturers to scientifically analyze and test their products for cellular efficacy with an aim to create the most efficacious combinations. This scientific approach will increase the credibility of the natural health industry in providing efficacy-based products and empower customers to choose supplements according to their needs instead of relying solely on marketing claims.
References
- Chen WC, Hsieh SR, Chiu CH, Hsu BD, Liou YM. Molecular identification for epigallocatechin-3-gallate-mediated antioxidant intervention on the H2O2-induced oxidative stress in H9c2 rat cardiomyoblasts. Journal of Biomedical Science. 2014; 21: 56.
- Kook D, Wolf AH, Yu AL, et al. The Protective Effect of Quercetin against Oxidative Stress in the Human RPE in Vitro. Investigative Ophthalmology & Visual Science. 2008; 49: 4.
- T.M. Vance, G. Azabdaftari, E.A. Pop, S.G. Lee, L.J. Su, E.T. Fontham , J.T. Bensen, S.E. Steck, L. Arab L, J.L. Mohler JL, M.H. Chen MH, S.I. Koo, O.K. Chun OK. Intake of dietary antioxidants is inversely associated with biomarkers of oxidative stress among men with prostate cancer. British Journal of Nutrition. 2016; 115(1):68-74.
- Turner TT, Lysiak JJ. Oxidative Stress: A Common Factor in Testicular Dysfunction. Journal of Andrology. 2008; September-October 29(5): 488–498.
- Sánchez A, Calpena AC, Clares B. Evaluating the Oxidative Stress in Inflammation: Role of Melatonin. International Journal of Molecular Sciences. 2015; 16(8): 16981–17004.
- Patel VP, Chu CT. Nuclear transport, oxidative stress, and neurodegeneration. International Journal of Clinical and Experimental Pathology. 2011; 4(3): 215-229.
- Use of Dietary Supplements www.cdc.gov/nchs/data/nhanes/databriefs/dietary.pdf
- Mensink GB, Fletcher R, Gurinovic M, et al. Mapping low intake of micronutrients across Europe. British Journal of Nutrition. 2012; 14: 1-19.
- Beitz R, Mensink GB, Rams S, et al. Vitamin- und Mineralstoffsupplementierung in Deutschland (Use of vitamin and mineral supplements in Germany). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz.2004; 47: 1057–1065.
- Tetens I, Biltoft-Jensen A, Spagner C, et al. Intake of micronutrients among Danish adult users and non-users of dietary supplements. Food & Nutrition Research. 2011; 55: 7153.
- Kiely M. North/South Ireland Food Consumption Survey. Summary Report on Food and Nutrient Intakes, Anthropometry, Attitudinal Data & Physical Activity Patterns. Irish Universities Nutrition Alliance. 2001.
- Rovira MA, Grau M, Castañer O, et al. Dietary supplement use and health-related behaviors in a Mediterranean population. Journal of Nutrition Education and Behavior. 2013; 45(5):386-391.
- Deutsch JC. Ascorbic acid oxidation by hydrogen peroxide. Analytical Biochemistry. 1998; 255(1): 1-7.
- Hajiaghaalipour F, Kanthimathi MS, Sanusi J, Rajarajeswaran J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chemistry. 2015; 169: 401-10.
- Lauridsen C, Engel H, Jensen SK, Craig AM, Traber MG. Lactating Sows and Suckling Piglets Preferentially Incorporate RRR- over All-rac-α-Tocopherol into Milk, Plasma and Tissues. Journal of Nutrition. 2002; 132 (6): 1258-1264.
- Disintegration and Dissolution of Dietary Supplements . The United States Pharmacopeial Convention. 2010 March 1
Table 1: Ingredient composition of tested supplements. The numbers of different vitamins, minerals, active compounds, amino acids or plant extracts in each product are listed in parenthesis. Different forms of a particular vitamin, for instance, mineral forms of Ascorbate and fat soluble form of Ascorbate, were counted as two distinct vitamins.

Fig. 1: Antioxidant capacity of tested supplements organized according to sets as described in Material and Methods. Results shown are an average of three separate experiments. All products were tested at 7 millionth part daily dose. Products manufactured by other companies are represented by shaded bars while our own formulations are represented by solid bars.
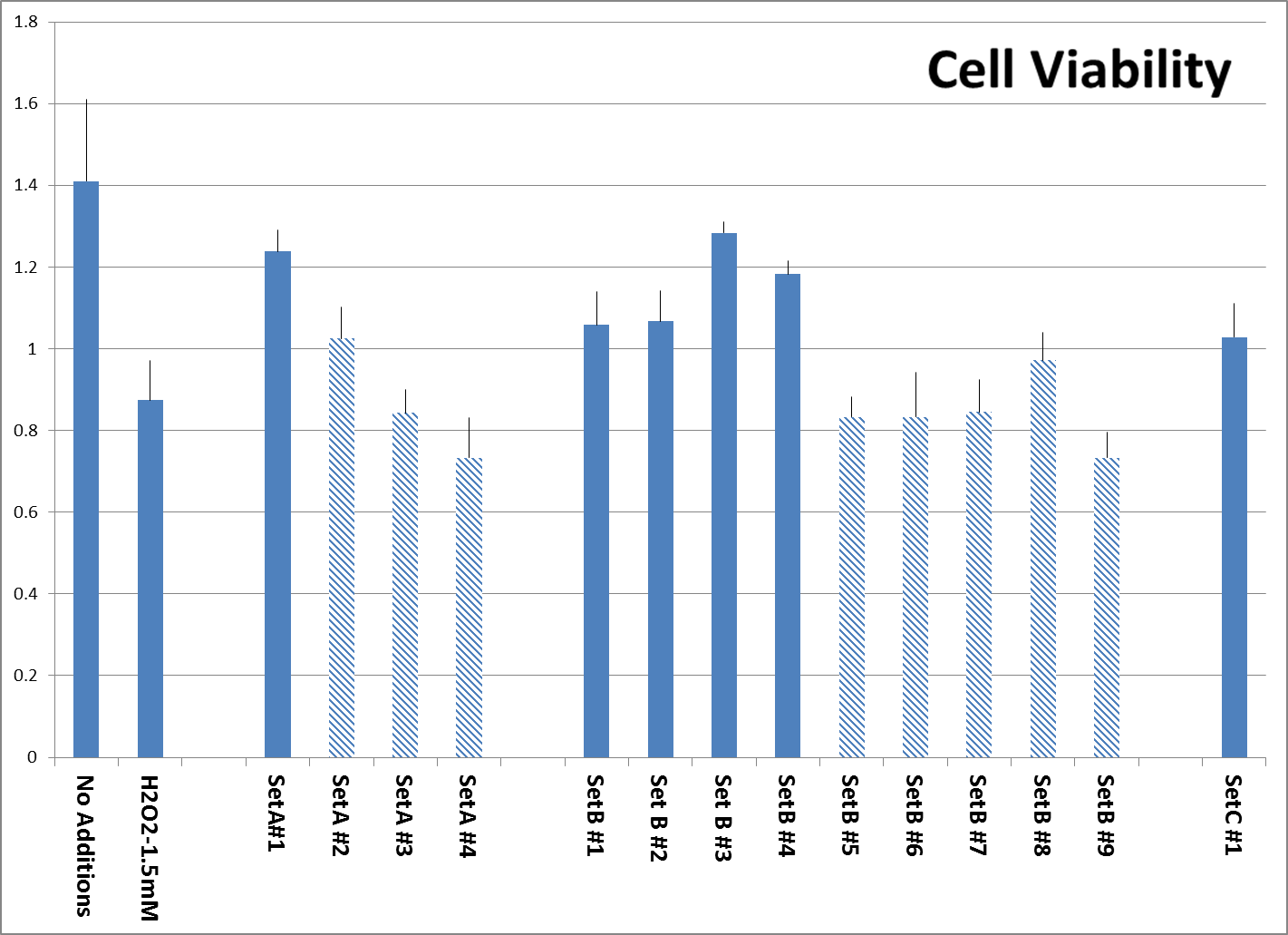
Fig. 2: Viability of additional surviving Human Aortic Smooth Muscle cells after pre-treatment with tested supplements, followed by H2O2 treatment as described, organized according to sets as described. Experiment was performed in triplicate. All products were tested at 0.25 millionth part of recommended daily dose. Viable cells reduce MTT reagent to a formazan product, which is estimated by optical density. Products manufactured by other companies are represented by shaded bars while both controls and our own formulations are represented by solid bars.

Fig. 3: Total Protein biomass of surviving Human Embryonic Kidney cells after pre-treatment with tested supplements, followed by H2O2 treatment as described, organized according to sets. Experiment was performed in quadruplicates. All products were tested at 0.25 millionth part daily dose. The bar labelled H2O2 1mM indicates the unprotected control which left only 28.2% of live protein biomass. Products manufactured by other companies are represented by shaded bars while both controls and our own formulations are represented by solid bars.
- Chen WC, Hsieh SR, Chiu CH, Hsu BD, Liou YM. Molecular identification for epigallocatechin-3-gallate-mediated antioxidant intervention on the H2O2-induced oxidative stress in H9c2 rat cardiomyoblasts. Journal of Biomedical Science. 2014; 21: 56.
- Kook D, Wolf AH, Yu AL, et al. The Protective Effect of Quercetin against Oxidative Stress in the Human RPE in Vitro. Investigative Ophthalmology & Visual Science. 2008; 49: 4.
- T.M. Vance, G. Azabdaftari, E.A. Pop, S.G. Lee, L.J. Su, E.T. Fontham , J.T. Bensen, S.E. Steck, L. Arab L, J.L. Mohler JL, M.H. Chen MH, S.I. Koo, O.K. Chun OK. Intake of dietary antioxidants is inversely associated with biomarkers of oxidative stress among men with prostate cancer. British Journal of Nutrition. 2016; 115(1):68-74.
- Turner TT, Lysiak JJ. Oxidative Stress: A Common Factor in Testicular Dysfunction. Journal of Andrology. 2008; September-October 29(5): 488–498.
- Sánchez A, Calpena AC, Clares B. Evaluating the Oxidative Stress in Inflammation: Role of Melatonin. International Journal of Molecular Sciences. 2015; 16(8): 16981–17004.
- Patel VP, Chu CT. Nuclear transport, oxidative stress, and neurodegeneration. International Journal of Clinical and Experimental Pathology. 2011; 4(3): 215-229.
- Use of Dietary Supplements www.cdc.gov/nchs/data/nhanes/databriefs/dietary.pdf
- Mensink GB, Fletcher R, Gurinovic M, et al. Mapping low intake of micronutrients across Europe. British Journal of Nutrition. 2012; 14: 1-19.
- Beitz R, Mensink GB, Rams S, et al. Vitamin- und Mineralstoffsupplementierung in Deutschland (Use of vitamin and mineral supplements in Germany). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz.2004; 47: 1057–1065.
- Tetens I, Biltoft-Jensen A, Spagner C, et al. Intake of micronutrients among Danish adult users and non-users of dietary supplements. Food & Nutrition Research. 2011; 55: 7153.
- Kiely M. North/South Ireland Food Consumption Survey. Summary Report on Food and Nutrient Intakes, Anthropometry, Attitudinal Data & Physical Activity Patterns. Irish Universities Nutrition Alliance. 2001.
- Rovira MA, Grau M, Castañer O, et al. Dietary supplement use and health-related behaviors in a Mediterranean population. Journal of Nutrition Education and Behavior. 2013; 45(5):386-391.
- Deutsch JC. Ascorbic acid oxidation by hydrogen peroxide. Analytical Biochemistry. 1998; 255(1): 1-7.
- Hajiaghaalipour F, Kanthimathi MS, Sanusi J, Rajarajeswaran J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chemistry. 2015; 169: 401-10.
- Lauridsen C, Engel H, Jensen SK, Craig AM, Traber MG. Lactating Sows and Suckling Piglets Preferentially Incorporate RRR- over All-rac-α-Tocopherol into Milk, Plasma and Tissues. Journal of Nutrition. 2002; 132 (6): 1258-1264.
- Disintegration and Dissolution of Dietary Supplements . The United States Pharmacopeial Convention. 2010 March 1

